Abstract
Objectives
This study was designed to assess the prevalence of adverse drug reactions (ADRs) in the internal medicine wards of two teaching Hospitals, identify the most common ADRs, the principal medications involved, and determine the risk factors implicated in the occurrence of such ADRs.
Methods
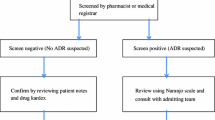
All admissions over 10 weeks were followed prospectively using an intensive drug surveillance method to identify ADRs. Clinical laboratory data, the drug prescribed, and ADRs were taken into consideration. Status of nutrition, liver and kidney function at admission, and ADR time were determined. In order to assess drug interactions a software package was used.
Results
A total of 405 patients were evaluated, 126 patients (31%) had 128 ADRs, 122 ADRs occurred during hospitalization. Two ADR-related deaths were observed during the study. Reactions affecting the gastrointestinal tract, skin, and hematological system were among the most frequent ADRs. For ADRs observed during admission predictors of its occurrence in a multivariate regression model were: OR (95% CI); more than 12 days’ hospitalization: 2.11(1.27–3.47), any drug interaction: 9.33 (5.12–17) and acute change in estimated glomerular filtration rate over admission >20%: 2.46 (1.45–4.2). Worsening of renal function or drug interaction was observed in nine of the ten ADRs. Age, sex, nutrition, and number of drugs used were not related to ADRs.
Conclusion
A significant prevalence of ADRs was found among hospitalized patients. Duration of hospital admission, changes in renal status during hospitalization and drug interactions seem to be important risk factors for ADRs.

Similar content being viewed by others
References
Kohn LT, Corrigan JM, Donaldson MS (eds) (2000) Institute of medicine. To err is human: building a safer health system. National Academies Press, Washington, DC
Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK (1998) Adverse drug reaction. BMJ 316:1295–1298
Davies EC, Green CF, Mottram DR, Pirmohamed M (2007) Adverse drug reactions in hospitals: a narrative review. Curr Drug Saf 2:79–87
Trontell A (2004) Expecting the unexpected: drug safety, pharmacovigilance, and the prepared mind. N Engl J Med 351:1385–1387
Talley RB, Laventurier MF (1975) Drug-induced illness. JAMA 229:1043–1048
Lagnaoui R, Moore N (2000) Adverse drug reactions in a department of systemic diseases-oriented internal medicine: prevalence, incidence, direct costs and avoidability. Eur J Clin Pharmacol 56:181–186
McDonnell PJ, Jacobs MR (2002) Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother 36:1331–1336
Klein U, Klein M, Sturm H (1976) The frequency of adverse drug reactions as dependent upon age, sex and duration of hospitalization. Int J Clin Pharmacol Biopharm 13:187–195
Martin RM, Biswas PN, Freemantle SN (1998) Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol 46:505–511
Zopf Y, Rabe C, Neubert A, Janson C, Brune K, Hahn EG, Dormann H (2009) Gender-based differences in drug prescription: relation to adverse drug reactions. Pharmacology 84:333–339
Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S (2007) Drug-related problems in hospitals: a review of the recent literature. Drug Saf 30:379–407
Schein JR (1998) Epidemiology, outcomes research, and drug interactions. Drug Metabol Drug Interact 14:147–158
Kuperman GJ, Bobb A, Payne TH (2007) Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc 14:29–40
Rawlins MD, Thompson JW (1991) Mechanisms of adverse drug reactions. In: Davies DM (ed) Textbook of adverse drug reactions. Oxford University Press, Oxford, pp 18–45
Naranjo CA, Busto U, Sellers EM et al (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245
Lexi-Comp (2010) Drug interactions handbook, and drug interactions software. http://www.lexi.com/web/index.jsp. Accessed June 2010)
Hazell L, Shakir SA (2006) Under-reporting of adverse drug reactions: a systematic review. Drug Saf 29:385–396
Pomeranz LJ, Corey PN BH (1998) A meta-analysis of prospective studies incidence of adverse drug reactions in hospitalized. JAMA 279:1200–1205
Brvar M, Fokter N, Bunc M, Mozina M (2009) The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol 9:8
Zopf Y, Rabe C, Neubert A, Hahn EG, Dormann H (2008) Risk factors associated with adverse drug reactions following hospital admission: a prospective analysis of 907 patients in two German university hospitals. Drug Saf 31:789–798
Camargo AL, Cardoso Ferreira MB, Heineck I (2006) Adverse drug reactions: a cohort study in internal medicine units at a university hospital. Eur J Clin Pharmacol 62:143–149
Fattinger K, Roos M, Vergères P (2000) Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 49:158–167
Bond CA, Raehl CL (2006) Adverse drug reactions in United States hospitals. Pharmacotherapy 26:601–608
Bates DW, Cullen DJ, Laird N et al (1995) Incidence of adverse drug events and potential drug events. Implications for prevention. JAMA 274:29–34
Flockhart DA (1995) Drug interactions and cytochrome P450 system: the role of cytochrome P450 2C19. Clin Pharmacokinet 29 [Suppl 1]:45–52
Szekendi MK, Sullivan C, Bobb A et al (2006) Active surveillance using electronic triggers to detect adverse events in hospitalized patients. Qual Saf Health Care 15:184–190
Bobb A, Gleason K, Husch M (2004) The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med 164:785–792
Helldén A, Bergman U, von Euler M (2009) Adverse drug reactions and impaired renal function in elderly patients admitted to the emergency department: a retrospective study. Drugs Aging 26:595–606
Verbeeck RK, Musuamba FT (2009) Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol 65:757–773
Lundkvist J, Jönsson B (2004) Pharmacoeconomics of adverse drug reactions. Fundam Clin Pharmacol 18:275–280
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez Muñoz-Torrero, J.F., Barquilla, P., Velasco, R. et al. Adverse drug reactions in internal medicine units and associated risk factors. Eur J Clin Pharmacol 66, 1257–1264 (2010). https://doi.org/10.1007/s00228-010-0866-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0866-6