Abstract
Purpose
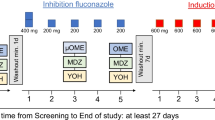
To investigate if the ordinary use of a vaginal suppository containing miconazole results in systemic absorption that is sufficient to affect the activities of CYP1A2 and CYP3A4, which are major drug- and steroid-metabolising enzymes.
Methods
In 20 healthy non-pregnant women aged 18–45 years, the serum concentration of miconazole was determined following the use of a vaginal suppository containing 1,200 mg miconazole. Enzyme activities of CYP1A2 and CYP3A4 were determined as metabolic ratios of caffeine (CMR = (AFMU + 1MU + 1MX)/17DMU) and quinidine (QMR = 3-hydroxy-quinidine/quinidine) respectively before and 34 h after insertion of the suppository. Miconazole was analysed by LC-MS/MS, while caffeine and metabolites were analysed by HPLC-UV and quinidine and hydroxy-quinidine were analysed by HPLC fluorescence.
Results
All 20 women had measurable concentrations of miconazole in serum (mean ± SD: 12.9 ± 5.6 μg/L; range: 3.5–24.6 μg/L). Although not statistically significant, an association between the serum concentrations of miconazole and the inhibition of CYP1A2 activity was indicated. No relation was observed between the CYP3A4 activity and the miconazole serum concentration.
Conclusions
Miconazole is absorbed via the vaginal mucosa to the systemic circulation in measurable concentrations. Our data indicate a concentration-dependent inhibition of CYP1A2, but the effect is negligible compared with the variation in the activity of CYP1A2 and is regarded to be of no clinical significance to the women. However, further studies on the ability of miconazole to be transferred across the placenta or to interfere with the placental function are warranted to secure safe use during pregnancy.





Similar content being viewed by others
References
Boelaert J, Daneels R, Van Landuyt H, Symoens J (1976) Miconazole plasma levels in healthy subjects and in patients with impaired renal function. Chemotherapy 6:165–169
Daneshmend TK, Warnock DW (1983) Clinical pharmacokinetics of systemic antifungal drugs. Clin Pharmacokinet 8:17–42
Daneshmend TK (1986) Systemic absorption of miconazole from the vagina. J Antimicrob Chemother 18:507–511
Mannisto PT, Mantyla R, Nykanen S, Lamminsivu U, Ottoila P (1982) Impairing effect of food on ketoconazole absorption. Antimicrob Agents Chemother 21:730–733
Stevens DA, Levine HB, Deresinski SC (1976) Miconazole in coccidioidomycosis. II. Therapeutic and pharmacologic studies in man. Am J Med 60:191–202
Murty M (2001) Miconazole-warfarin interaction: increased INR. CMAJ 165:81–86
Pemberton MN, Oliver RJ, Theaker ED (2004) Miconazole oral gel and drug interactions. Br Dent J 196:529–531
Kjaerstad MB, Taxvig C, Nellemann C, Vinggaard AM, Andersen HR (2010) Endocrine disrupting effects in vitro of conazole antifungals used as pesticides and pharmaceuticals. Reprod Toxicol. doi:10.1016/j.reprotox.2010.07.009
Ayub M, Levell MJ (1987) Inhibition of testicular 17 alpha-hydroxylase and 17, 20-lyase but not 3 beta-hydroxysteroid dehydrogenase-isomerase or 17 beta-hydroxysteroid oxidoreductase by ketoconazole and other imidazole drugs. J Steroid Biochem 28:521–531
Ayub M, Levell MJ (1989) Inhibition of human adrenal steroidogenic enzymes in vitro by imidazole drugs including ketoconazole. J Steroid Biochem 32:515–524
Mason JI, Carr BR, Murry BA (1987) Imidazole antimycotics: selective inhibitors of steroid aromatization and progesterone hydroxylation. Steroids 50:179–189
Trosken ER, Fischer K, Volkel W, Lutz WK (2006) Inhibition of human CYP19 by azoles used as antifungal agents and aromatase inhibitors, using a new LC-MS/MS method for the analysis of estradiol product formation. Toxicology 219:33–40
Trosken ER, Adamska M, Arand M et al (2006) Comparison of lanosterol-14 alpha-demethylase (CYP51) of human and Candida albicans for inhibition by different antifungal azoles. Toxicology 228:24–32
Zhang W, Ramamoorthy Y, Kilicarslan T et al (2002) Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metab Dispos 30:314–318
Shou M, Korzekwa KR, Brooks EN et al (1997) Role of human hepatic cytochrome P450 1A2 and 3A4 in the metabolic activation of estrone. Carcinogenesis 18:207–214
Yamazaki H, Shaw PM, Guengerich FP, Shimada T (1998) Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol 11:659–665
Grandjean P, Bellinger D, Bergman A et al (2008) The Faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol 102:73–75
Skakkebaek NE, Rajpert-De Meyts E, Main KM (2001) Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16:972–978
Swan SH, Main KM, Liu F et al (2005) Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 113:1056–1061
Taxvig C, Hass U, Axelstad M et al (2007) Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol Sci 100:464–473
Vinggaard AM, Hass U, Dalgaard M et al (2006) Prochloraz: an imidazole fungicide with multiple mechanisms of action. Int J Androl 29:186–192
Christensen M, Andersson K, Dalen P et al (2003) The Karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther 73:517–528
Damkier P, Brosen K (2000) Quinidine as a probe for CYP3A4 activity: intrasubject variability and lack of correlation with probe-based assays for CYP1A2, CYP2C9, CYP2C19, and CYP2D6. Clin Pharmacol Ther 68:199–209
Frye RF, Matzke GR, Adedoyin A, Porter JA, Branch RA (1997) Validation of the five-drug “Pittsburgh cocktail” approach for assessment of selective regulation of drug-metabolising enzymes. Clin Pharmacol Ther 62:365–376
Rost KL, Roots I (1994) Accelerated caffeine metabolism after omeprazole treatment is indicated by urinary metabolite ratios: coincidence with plasma clearance and breath test. Clin Pharmacol Ther 55:402–411
Rasmussen BB, Brosen K (1996) Determination of urinary metabolites of caffeine for the assessment of cytochrome P4501A2, xanthine oxidase, and N-acetyltransferase activity in humans. Ther Drug Monit 18:254–262
Nielsen F, Nielsen KK, Brosen K (1994) Determination of quinidine, dihydroquinidine, (3S)-3-hydroxyquinidine and quinidine N-oxide in plasma and urine by high-performance liquid chromatography. J Chromatogr B Biomed Appl 660:103–110
Nielsen JB (2005) Percutaneous penetration through slightly damaged skin. Arch Dermatol Res 296:560–567
Duret C, Daujat-Chavanieu M, Pascussi JM et al (2006) Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor. Mol Pharmacol 70:329–339
Rasmussen BB, Brix TH, Kyvik KO, Brosen K (2002) The interindividual differences in the 3-demethylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics 12:473–478
Petersen MS, Halling J, Damkier P et al (2006) Caffeine N3-demethylation (CYP1A2) in a population with an increased exposure to polychlorinated biphenyls. Eur J Clin Pharmacol 62:1041–1048
Kashuba AD, Bertino JS Jr, Kearns GL et al (1998) Quantitation of three-month intraindividual variability and influence of sex and menstrual cycle phase on CYP1A2, N-acetyltransferase-2, and xanthine oxidase activity determined with caffeine phenotyping. Clin Pharmacol Ther 63:540–551
Kharasch ED, Russell M, Garton K et al (1997) Assessment of cytochrome P450 3A4 activity during the menstrual cycle using alfentanil as a noninvasive probe. Anesthesiology 87:26–35
Zaigler M, Rietbrock S, Szymanski J et al (2000) Variation of CYP1A2-dependent caffeine metabolism during menstrual cycle in healthy women. Int J Clin Pharmacol Ther 38:235–244
Petersen MS, Halling J, Damkier P et al (2007) Polychlorinated biphenyl (PCB) induction of CYP3A4 enzyme activity in healthy Faroese adults. Toxicol Appl Pharmacol 224:202–206
Chang C, Bahadduri PM, Polli JE, Swaan PW, Ekins S (2006) Rapid identification of P-glycoprotein substrates and inhibitors. Drug Metab Dispos 34:1976–1984
Elsby R, Surry DD, Smith VN, Gray AJ (2008) Validation and application of Caco-2 assays for the in vitro evaluation of development candidate drugs as substrates or inhibitors of P-glycoprotein to support regulatory submissions. Xenobiotica 38:1140–1164
Fromm MF, Kim RB, Stein CM, Wilkinson GR, Roden DM (1999) Inhibition of P-glycoprotein-mediated drug transport: a unifying mechanism to explain the interaction between digoxin and quinidine [see comments]. Circulation 99:552–557
Sakaeda T, Iwaki K, Kakumoto M et al (2005) Effect of micafungin on cytochrome P450 3A4 and multidrug resistance protein 1 activities, and its comparison with azole antifungal drugs. J Pharm Pharmacol 57:759–764
Vahakangas K, Myllynen P (2009) Drug transporters in the human blood-placental barrier. Br J Pharmacol 158:665–678
Niwa T, Shiraga T, Takagi A (2005) Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull 28:1805–1808
Niwa T, Inoue-Yamamoto S, Shiraga T, Takagi A (2005) Effect of antifungal drugs on cytochrome P450 (CYP) 1A2, CYP2D6, and CYP2E1 activities in human liver microsomes. Biol Pharm Bull 28:1813–1816
Ariyaratnam S, Thakker NS, Sloan P, Thornhill MH (1997) Potentiation of warfarin anticoagulant activity by miconazole oral gel. BMJ 314:349
O'Reilly RA, Goulart DA, Kunze KL et al (1992) Mechanisms of the stereoselective interaction between miconazole and racemic warfarin in human subjects. Clin Pharmacol Ther 51:656–667
Silingardi M, Ghirarduzzi A, Tincani E, Iorio A, Iori I (2000) Miconazole oral gel potentiates warfarin anticoagulant activity. Thromb Haemost 83:794–795
Acknowledgements
This work was supported by Clinical Pharmacology and the Department of Environmental Medicine at the University of Southern Denmark and with grants from Hartmann Fonden (number A1461), Beckett Fonden (number 80530), and Kong Christian den tiendes Fond (number 75/2008).
We are indebted to Vibeke Kvist Pedersen and Stine Rosendahl for excellent technical assistance.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical trial registry: www.clinicaltrials.gov
Registration number: NCT00668538
Rights and permissions
About this article
Cite this article
Kjærstad, M.B., Nielsen, F., Nøhr-Jensen, L. et al. Systemic uptake of miconazole during vaginal suppository use and effect on CYP1A2 and CYP3A4 associated enzyme activities in women. Eur J Clin Pharmacol 66, 1189–1197 (2010). https://doi.org/10.1007/s00228-010-0906-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0906-2