Abstract
Purpose
Oral contraceptive pills (OCPs) are an important element of hepatitis C virus (HCV) treatment in women of childbearing potential. These studies evaluated the safety and pharmacokinetic interactions between elbasvir (EBR) and grazoprevir (GZR) and ethinyl estradiol/levonorgestrel (EE/LNG).
Methods
Both studies were open-label, single-site, two-period, fixed-sequence, one-way interaction studies. In period 1, subjects received one tablet of EE/LNG (0.03 mg/0.15 mg). In period 2, subjects received EBR (50 mg once daily) for 13 days or GZR (200 mg once daily) for 10 days, with one tablet of EE/LNG on day 7 (GZR group) or 10 (EBR group). Each study enrolled 20 healthy, nonsmoking adult females.
Results
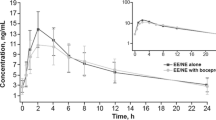
There was no clinically meaningful effect of multiple doses of EBR or GZR on the pharmacokinetics of EE or LNG. Geometric mean ratios (GMRs) for AUC0–∞ and Cmax in the presence and absence of EBR were 1.01 and 1.10 for EE and 1.14 and 1.02 for LNG, with 90% confidence intervals (CIs) that were contained in the interval [0.80, 1.25]. Similarly, the AUC0–∞ and Cmax GMRs in the presence and absence of GZR were 1.10 and 1.05 for EE and 1.23 and 0.93 for LNG, respectively. The 90% CIs for EE AUC0–∞ and for EE and LNG Cmax were contained in the interval [0.80, 1.25]; however, the 90% CI for the LNG AUC0–∞ [1.15, 1.32] slightly exceeded the upper bound.
Conclusions
These results suggest that EBR/GZR can be co-administered to female patients with HCV of childbearing potential who are on OCPs to prevent pregnancy.


Similar content being viewed by others
References
Lavanchy D (2011) Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect 17:107–115
Chen SL, Morgan TR (2006) The natural history of hepatitis C virus (HCV) infection. Int J Med Sci 3:47–52
AASLD/IDSA HCV Guidance Panel (2015) Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 62:932–954
Merck & Co., Inc. (2016) Zepatier™ (elbasvir and grazoprevir): US prescribing information https://www.merck.com/product/usa/pi_circulars/z/zepatier/zepatier_pi.pdf. Accessed on 8 Sept 2016
Zepatier [elbasvir/grazoprevir] European Medicines Agency (2016) http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/004126/WC500211238.pdf. Accessed 19 Oct 2016
Zeuzem S, Ghalib R, Reddy KR, Pockros PR, Ben Ari Z, Zhao Y, Brown DD, Wan S, DiNubile MJ, Nguyen B-Y, Robertson MN, Wahl J, Barr E, Butterton JR (2015) Grazoprevir–elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic HCV genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 163:1–13
Forns X, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, Gilbert C, Placza J, Howe AYM, DiNubile MJ, Robertson MN, Wahl J, Barr E, Buti M (2015) Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent. J Hepatol 63:564–572
Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, Matthews GV, Saag MS, Zamor PJ, Orkin C, Gress J, Klopfer S, Shaughnessy M, Wahl J, Nguyen B-YT, Barr E, Platt HL, Robertson MN, Sulkowski M (2015) Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV 2:e319–e327
Roth D, Nelson DR, Bruchfeld A, Liapakis AM, Silva M, Monsour H Jr, Martin P, Pol S, Londoño M-C, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen B-Y, Robertson M, Barr E, Wahl J, Greaves W (2015) Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 386:1537–1545
Roberts SS (2008) Assessing ribavirin exposure during pregnancy: the Ribavirin Pregnancy Registry. Gastroenterol Nurs 31:413–417
Ament PW, Bertolino JG, Liszewski JL (2000) Clinically significant drug interactions. Am Fam Physician 61:1745–1754
Zhang H, Cui D, Wang B, Han YH, Balimane P, Yang Z, Sinz M, Rodrigues AD (2007) Pharmacokinetic drug interactions involving 17alpha-ethinylestradiol: a new look at an old drug. Clin Pharmacokinet 46:133–157
Benedetti MS (2000) Enzyme induction and inhibition by new antiepileptic drugs: a review of human studies. Fundam Clin Pharmacol 14:301–319
Hall SD, Wang Z, Huang S-M, Hamman MA, Vasavada N, Adigun AQ, Hilligoss JK, Miller M, Gorski JC (2003) The interaction between St John’s wort and an oral contraceptive. Clin Pharmacol Ther 74:525–535
Fattore C, Cipolla G, Gatti G, Limido GL, Sturm Y, Bernasconi C, Perucca E (1999) Induction of ethinylestradiol and levonorgestrel metabolism by oxcarbazepine in healthy women. Epilepsia 40:783–787
Fotherby K (1995) Levonorgestrel. Clinical pharmacokinetics. Clin Pharmacokinet 28:203–215
Teva Womens Health, Inc. (2016) NORDETTE®-28 (levonorgestrel and ethinyl estradiol tablets, 0.15 mg/0.03 mg): US presecribing information http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/018782s036lbl.pdf. Accessed on 8 Sept 2016
Duramed Pharmaceuticals, Inc. (2010) Seasonique (levonorgestrel/ethinyl estradiol tablets and ethinyl estradiol tablets): US prescribing information (https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021840s008lbl.pdf. Accessed 8 Sept 2016
AbbVie, Inc. (2016) VIEKIRA PAK® (ombitasvir, paritaprevir, and ritonavir tablets; dasabuvir tablets): US prescribing information http://www.rxabbvie.com/pdf/viekirapak_pi.pdf. Accessed 8 Sept 2016.
Acknowledgments
The authors thank all the subjects and clinical research unit staff who participated in this study. The authors also thank Monica Martinho of Merck and Nadia Cardillo Marricco, Daria Stypinski, Xiaopeng Li, and Bruce DeGroot of Celerion for their assistance in this study. This assistance was funded by Merck & Co., Inc., Kenilworth, NJ, USA. Medical writing and editorial assistance was provided by Sally-Anne Mitchell, PhD, and Tim Ibbotson, PhD, of ApotheCom (Yardley, PA, USA) and funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author’s contribution
Conception, design, or planning of the study: JB, LC, ZG, XH, JM, WM, TR, DP, JT, WY.
Acquisition of the data: ZG, JM, WM, TR, WY.
Analysis of the data: H-PF, ZG, XH, EM, WM, WY.
Interpretation of the results: JB, LC, H-PF, ZG, JM, WM, JT, WY.
Drafting of the manuscript: ZG, WM, WY.
Critically reviewing or revising the manuscript for important intellectual content: JB, LC, H-PF, ZG, XH, JM, EM, WM, TR, DP, JT, WY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
WWY, CBS, LC, HPF, JT, JM, EM, XH, RV, ZG, and JRB are current employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. WLM was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, at the time the studies were conducted. TR has nothing to disclose.
Informed consent
All of the subjects gave their written consent to their participation in the study.
Funding
The study was funded by Merck & Co., Inc.
Rights and permissions
About this article
Cite this article
Marshall, W.L., Feng, HP., Caro, L. et al. No clinically meaningful pharmacokinetic interaction between the hepatitis C virus inhibitors elbasvir and grazoprevir and the oral contraceptives ethinyl estradiol and levonorgestrel. Eur J Clin Pharmacol 73, 593–600 (2017). https://doi.org/10.1007/s00228-017-2216-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2216-4