Abstract
Introduction
Haematoma and oedema size determines outcome after intracerebral haemorrhage (ICH), with each added 10 % volume increasing mortality by 5 %. We assessed the reliability of semi-automated computed tomography planimetry using Analyze and Osirix softwares.
Methods
We randomly selected 100 scans from 1329 ICH patients from two centres. We used Hounsfield Unit thresholds of 5–33 for oedema and 44–100 for ICH. Three raters segmented all scans using both softwares and 20 scans repeated for intra-rater reliability and segmentation timing. Volumes reported by Analyze and Osirix were compared to volume estimates calculated using the best practice method, taking effective individual slice thickness, i.e. voxel depth, into account.
Results
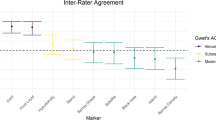
There was excellent overall inter-rater, intra-rater and inter-software reliability, all intraclass correlation coefficients >0.918. Analyze and Osirix produced similar haematoma (mean difference: Analyze − Osirix = 1.5 ± 5.2 mL, 6 %, p ≤ 0.001) and oedema volumes (−0.6 ± 12.6 mL, −3 %, p = 0.377). Compared to a best practice approach to volume calculation, the automated haematoma volume output was 2.6 mL (−11 %) too small with Analyze and 4.0 mL (−18 %) too small with Osirix, whilst the oedema volumes were 2.5 mL (−12 %) and 5.5 mL (−25 %) too small, correspondingly. In scans with variable slice thickness, the volume underestimations were larger, −29%/−36 % for ICH and −29 %/−41 % for oedema. Mean segmentation times were 6:53 ± 4:02 min with Analyze and 9:06 ± 5:24 min with Osirix (p < 0.001).
Conclusion
Our results demonstrate that the method used to determine voxel depth can influence the final volume output markedly. Results of clinical and collaborative studies need to be considered in the context of these methodological differences.




Similar content being viewed by others
References
Hemphill JC 3rd, Greenberg SM, Anderson CS et al (2015) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46(7):2032–2060
Steiner T, Al-Shahi Salman R, Beer R et al (2014) European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 9(7):840–855
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G (1993) Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24(7):987–993
Urday S, Kimberly WT, Beslow LA et al (2015) Targeting secondary injury in intracerebral haemorrhage–perihaematomal oedema. Nat Rev Neurol 11(2):111–122
Yang J, Arima H, Wu G et al (2015) Prognostic significance of perihematomal edema in acute intracerebral hemorrhage: pooled analysis from the intensive blood pressure reduction in acute cerebral hemorrhage trial studies. Stroke 46(4):1009–1013
Yeatts SD, Palesch YY, Moy CS, Selim M (2013) High Dose Deferoxamine in Intracerebral Hemorrhage (HI-DEF) trial: rationale, design, and methods. Neurocrit Care 19(2):257–266
Fu Y, Hao J, Zhang N et al (2014) Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol 71(9):1092–1101
Kollmar R, Juettler E, Huttner HB et al (2012) Cooling in Intracerebral Hemorrhage (CINCH) trial: protocol of a randomized German–Austrian clinical trial. Int J Stroke 7(2):168–172
Gonzales NR, Shah J, Sangha N et al (2013) Design of a prospective, dose-escalation study evaluating the Safety of Pioglitazone for Hematoma Resolution in Intracerebral Hemorrhage (SHRINC). Int J Stroke 8(5):388–396
Volbers B, Staykov D, Wagner I et al (2011) Semi-automatic volumetric assessment of perihemorrhagic edema with computed tomography. Eur J Neurol 18(11):1323–1328
Urday S, Beslow LA, Goldstein DW et al (2015) Measurement of perihematomal edema in intracerebral hemorrhage. Stroke 46(4):1116–1119
Kosior JC, Idris S, Dowlatshahi D et al (2011) Quantomo: validation of a computer-assisted methodology for the volumetric analysis of intracerebral haemorrhage. Int J Stroke 6(4):302–305
McCourt R, Gould B, Gioia L et al (2014) Cerebral perfusion and blood pressure do not affect perihematoma edema growth in acute intracerebral hemorrhage. Stroke 45(5):1292–1298
Krishnan K, Mukhtar SF, Lingard J et al (2015) Performance characteristics of methods for quantifying spontaneous intracerebral haemorrhage: data from the Efficacy of Nitric Oxide in Stroke (ENOS) trial. J Neurol Neurosurg Psychiatry 86(11):1258–1266
Meretoja A, Strbian D, Putaala J et al (2012) SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke 43(10):2592–2597
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Kottner J, Audige L, Brorson S et al (2011) Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. Int J Nurs Stud 48(6):661–671
Rodriguez-Luna D, Boyko M, Subramaniam S et al (2016) Magnitude of hematoma volume measurement error in intracerebral hemorrhage. Stroke 47(4):1124–1126
Divani AA, Majidi S, Luo X et al (2011) The ABCs of accurate volumetric measurement of cerebral hematoma. Stroke 42(6):1569–1574
Mould WA, Carhuapoma JR, Muschelli J et al (2013) Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke 44(3):627–634. doi:10.1161/STROKEAHA.111.000411
Delcourt C, Huang Y, Arima H et al (2012) Hematoma growth and outcomes in intracerebral hemorrhage: the INTERACT1 Study. Neurology 79(4):314–319
Davis SM, Broderick J, Hennerici M et al (2006) Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66(8):1175–1181
Meretoja A, Churilov L, Campbell BC et al (2014) The spot sign and tranexamic acid on preventing ICH growth—AUStralasia Trial (STOP-AUST): protocol of a phase II randomized, placebo-controlled, double-blind, multicenter trial. Int J Stroke 9(4):519–524
Appelboom G, Bruce SS, Hickman ZL et al (2013) Volume-dependent effect of perihaematomal oedema on outcome for spontaneous intracerebral haemorrhages. J Neurol Neurosurg Psychiatry 84(5):488–493
Volbers B, Willfarth W, Kuramatsu JB et al (2016) Impact of perihemorrhagic edema on short-term outcome after intracerebral hemorrhage. Neurocrit Care 24(3):404–412
Acknowledgments
TYW is supported by grants from the Neurological Foundation of New Zealand and the Royal Melbourne Hospital Neuroscience Foundation. AP-J is supported by a National Institute for Health Research Clinician Scientist Award. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. AM is supported by grants from National Health and Medical Research Council (Australia), the Academy of Finland and the Finnish Medical Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human and animal studies have been approved by the Helsinki University Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that given no identifiable patient data is presented, Helsinki University Hospital waived informed consent for this observational registry study.
Conflict of interest
We declare that we have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table
Technical code of the workflow for volume calculation (DOCX 71 kb)
Supplementary Fig. 1
Effect of gantry tilt on voxel depth calculation. Two representative image slices of 7.5 mm thickness with a gantry tilt of 16.5°. Slice thickness is derived from midpoint to midpoint distance. a) Gantry tilt was not adjusted when image position patient (DICOM header, 0020, 0032) coordinates were used resulting in an inter-slice distance of 7.82 mm, solid line. B) Gantry tilt was adjusted using our in-house method and the inter-slice distance was 7.5 mm, dotted line (PDF 729 kb)
Supplementary Fig. 2
Bland–Altman plots for oedema segmentation. Inter-rater Bland–Altman plots with intraclass correlation coefficients (ICC) for semi-automated planimetry of oedema segmentation on Analyze and Osirix. The solid line represents mean volume difference between raters and the dotted lines are the limits of 95 % agreement. The outliers all had significant white matter changes. (PDF 320 kb)
ESM 4
(XLSX 22 kb)
ESM 5
(MOV 13673 kb)
ESM 6
(MOV 80349 kb)
ESM 7
(ZIP 15 kb)
Rights and permissions
About this article
Cite this article
Wu, T.Y., Sobowale, O., Hurford, R. et al. Software output from semi-automated planimetry can underestimate intracerebral haemorrhage and peri-haematomal oedema volumes by up to 41 %. Neuroradiology 58, 867–876 (2016). https://doi.org/10.1007/s00234-016-1720-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1720-z