Abstract
Purpose
Endovascular thrombectomy (EVT) improves clinical outcomes in ischemic stroke with large vessel occlusion. Clinical benefits are inversely proportional to size of the pre-treatment ischemic core. This study compared estimated ischemic core volumes by two different CT perfusion (CTP) automated algorithms to the gold standard follow-up infarct volume using diffusion-weighted imaging (DWI) to assess for congruence, and thus eligibility for EVT.
Methods
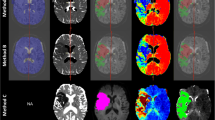
Retrospective, single-center cohort study of 102 patients presenting to a comprehensive stroke center between 2012 and 2018. Inclusion criteria were CT perfusion prior to EVT, successful EVT with mTIBI 2b-3 reperfusion, and DWI post-EVT. CTP data were retrospectively processed by two algorithms: “delay and dispersion insensitive deconvolution” (DISD, RAPID software) versus “delay and dispersion corrected single value decomposition” (ddSVD, Mistar software), using commercially available software. Core volumes were compared to follow up DWI using independent software (MRIcron). Agreement between each algorithm and DWI was estimated using Lin’s concordance coefficient and analyzed using reduced major axis regression.
Results
We included 102 patients. Both algorithms had excellent agreement with DWI (Lin’s concordance coefficients: DISD 0.8 (95% CI: 0.73; 0.87), ddSVD 0.92 (95% CI: 0.89; 0.95). Compared to ddSVD (reduced major axis slope = 0.95), DISD exhibited a larger extent of proportional bias (slope = 1.12).
Conclusion
The ddSVD algorithm better correlates with DWI follow-up infarct volume than DISD processing. The DISD algorithm overestimated larger ischemic cores which may lead to patient exclusion from thrombectomy based on selection by core volume.


Similar content being viewed by others
Data availability
Available upon request
References
Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, Fiehler J (2019) European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischemic Stroke. JNIS:neurintsurg-2018-014569. https://doi.org/10.1136/neurintsurg-2018-014569
Goyal M, Menon B, Zwam W, Dippel DWJ, Mitchell PJ, Demchuk AM et al (2016) Endovascular thrombectomy after large vessel ischemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387(10029):1723–1731
Powers W, Rabinstein A, Ackerson T, Adeoye OM, Bambakidis NC, Becker K et al (2018) 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(3):46–99
Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP, for the DEFUSE Investigators (2006) Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 60:508–517
Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG (2009) MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke 40:2046–2054
Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA et al (2010) Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab 30:1214–1225
Olivot JM, Mosimann PJ, Labreuche J, Inoue M, Meseguer E, Desilles JP, Rouchaud A, Klein IF, Straka M, Bammer R, Mlynash M, Amarenco P, Albers GW, Mazighi M (2013) Impact of diffusion-weighted imaging lesion volume on the success of endovascular reperfusion therapy. Stroke 44:2205–2211
Sarraj A, Hassan AE, Savitz S, Sitton C, Grotta J, Chen P, Cai C, Cutter G, Imam B, Reddy S, Parsha K, Pujara D, Riascos R, Vora N, Abraham M, Kamal H, Haussen DC, Barreto AD, Lansberg M, Gupta R, Albers GW (2019) Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient’s selection for endovascular treatment in acute ischemic stroke (SELECT) study. JAMA Neurol 76(10):1147–1156. https://doi.org/10.1001/jamaneurol.2019.2109
Kerenyi L, Kardos L, Szász J, Szatmari D, Bereczki D, Hegedus et al. Factors influencing hemorrhagic transformation in ischemic stroke: a clinicopathological comparison. Eur J Neurol 2006;13:1251-1255
Yu W, Jiang W (2019) A simple imaging guide for endovascular thrombectomy in acute ischemic stroke: from time window to perfusion mismatch and beyond. Front Neurol. https://doi.org/10.3389/fneur.2019.00502
Bivard A, Levi C, Spratt N, Parsons M (2013) Perfusion CT in acute stroke: a comprehensive analysis of infarct and penumbra. Radiology 267(2):543–550
Khaw AV, Angermaier A, Michel P, Kirsch M, Kessler C, Langner S (2016) Inter-rater agreement in three perfusion-computed tomography evaluation methods before endovascular therapy for acute ischemic stroke. J Stroke Cerebrovasc Dis 25:960–968. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.01.001
Mouannes-Srour JJ, Shin W, Ansari SA, Hurley MC, Vakil P, Bendok BR, Lee JL, Derdeyn CP, Carroll TJ (2012) Correction for arterial-tissue delay and dispersion in absolute quantitative cerebral perfusion DSC MR imaging. Magn Reson Med 68(2):495–506. https://doi.org/10.1002/mrm.23257
Lin L, Bivard A, Kleinig T, Spratt NJ, Levi CR, Qing Y et al (2018) Correction for delay and dispersion results in more accurate cerebral blood flow ischemic core measurement in acute stroke. Stroke 49(4):924–930
Bivard A, Kleinig T, Miteff F, Butcher K, Lin L, Levi C, Parsons M (2017) Ischemic core thresholds change with time to reperfusion: a case control study. Ann Neurol 82:995–1003. https://doi.org/10.1002/ana.25109
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart R, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG, DEFUSE 3 Investigators (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. NEJM 378(8):708–718
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics. 33(1):159–174
Walter SD, Eliasziw M, Donner A (1998) Sample size and optimal designs for reliability studies. Stat Med 17:101–110. https://doi.org/10.1002/(SICI)1097-0258(19980115)17:1<101::AID-SIM727>3.0.CO;2-E
Martins N, Aires A, Mendez B, Boned S, Rubiera M, Tomasello A, Coscojuela P, Hernandez D, Muchada M, Rodríguez-Luna D, Rodríguez N, Juega JM, Pagola J, Molina CA, Ribó M (2018) Ghost infarct core and admission computed tomography perfusion: redefining the role of neuroimaging in acute ischemic stroke. Intervent Neurol 7:513–521. https://doi.org/10.1159/000490117
Kranz PG, Eastwood JD (2009) Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review [published correction appears in AJNR Am J Neuroradiol. 2009 Aug;30(7):E114]. AJNR Am J Neuroradiol 30(6):1206–1212. https://doi.org/10.3174/ajnr.A1547
Schaefer PW, Souza L, Kamalian S, Hirsch JA, Yoo AJ, Kamalian S, Gonzalez RG, Lev MH (2015) Limited reliability of computed tomographic perfusion acute infarct volume measurements compared with diffusion-weighted imaging in anterior circulation stroke. Stroke. 46(2):419–424. https://doi.org/10.1161/STROKEAHA.114.007117
Inoue M, Mlynash M, Christensen S, Wheeler HM, Straka M, Tipimeni A et al (2014) Early diffusion-weighted imaging reversal after endovascular reperfusion is typically transient in patients imaged 3 to 6 hours after onset. Stroke. 45:1024–1028
Chemmanam T, Campbell BC, Christensen S, Nagakane Y, Desmond PM, Bladin CF et al (2010) EPITHET Investigators. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology. 75:1040–1047
Campbell BC, Purushotham A, Christensen S, Desmond PM, Nagakane Y, Parsons MW et al (2012) The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. J Cereb Blood Flow Metab 32:50–56
Rivers CS, Wardlaw JM, Armitage PA, Bastin ME, Carpenter TK, Cvoro V, Hand PJ, Dennis MS (2006) Persistent infarct hyperintensity on diffusion-weighted imaging late after stroke indicates heterogeneous, delayed, infarct evolution. Stroke. 37:1418–1423
Materials availability
Available upon request
Code availability
RAPID (Version 4.6.1, iSchemaView, MountainView, California) and Mistar (Version 3.2.63, Apollo Medical Imaging Technology, Melbourne, Australia) were used (see “Methods” section).
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
BY and MP conceived the study. LG performed data collection. LG, BY, MP, and AB analyzed data. LG drafted the manuscript and all authors contributed equally to its revision. LC, BY, MP, and AB were involved in the statistical analysis. LG and BY take responsibility for the paper as a whole.
Corresponding author
Ethics declarations
Ethics approval
Royal Melbourne Hospital Institutional Review Board approval (HREC QA 2013-072).
Consent to participate/consent for publication
Not applicable
Conflicts of interest
MP and AB have research partnerships with Apollo, Siemens, and Canon.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figure 1
Sample outcome from anonymised patient (PNG 3945 kb)
Rights and permissions
About this article
Cite this article
Gunasekera, L., Churilov, L., Mitchell, P. et al. Automated estimation of ischemic core prior to thrombectomy: comparison of two current algorithms. Neuroradiology 63, 1645–1649 (2021). https://doi.org/10.1007/s00234-021-02651-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02651-9