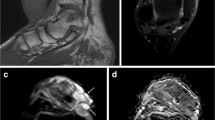
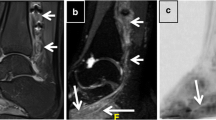
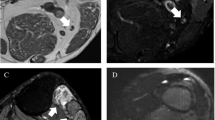
Abstract
Purpose
To perform a review of the physical basis of DTI and DCE-MRI applied to Peripheral Nerves (PNs) evaluation with the aim of providing readers the main concepts and tools to acquire these types of sequences for PNs assessment. The potential added value of these advanced techniques for pre-and post-surgical PN assessment is also reviewed in diverse clinical scenarios. Finally, a brief introduction to the promising applications of Artificial Intelligence (AI) for PNs evaluation is presented.
Methods
We review the existing literature and analyze the latest evidence regarding DTI, DCE-MRI and AI for PNs assessment. This review is focused on a practical approach to these advanced sequences providing tips and tricks for implementing them into real clinical practice focused on imaging postprocessing and their current clinical applicability. A summary of the potential applications of AI algorithms for PNs assessment is also included.
Results
DTI, successfully used in central nervous system, can also be applied for PNs assessment. DCE-MRI can help evaluate PN's vascularization and integrity of Blood Nerve Barrier beyond the conventional gadolinium-enhanced MRI sequences approach. Both approaches have been tested for PN assessment including pre- and post-surgical evaluation of PNs and tumoral conditions. AI algorithms may help radiologists for PN detection, segmentation and characterization with promising initial results.
Conclusion
DTI, DCE-MRI are feasible tools for the assessment of PN lesions. This manuscript emphasizes the technical adjustments necessary to acquire and post-process these images. AI algorithms can also be considered as an alternative and promising choice for PN evaluation with promising results.






Similar content being viewed by others
Change history
31 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00234-022-02936-7
References
Novak CB, Anastakis DJ, Beaton DE et al (2011) Biomedical and psychosocial factors associated with disability after peripheral nerve injury. J Bone Jt Surg - Ser A 93:929–936. https://doi.org/10.2106/JBJS.J.00110
Ciaramitaro P, Mondelli M, Logullo F et al (2010) Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst 15:120–127. https://doi.org/10.1111/j.1529-8027.2010.00260.x
Menorca RMG, Fussell TS, Elfar JC (2013) Nerve physiology. Mechanisms of injury and recovery. Hand Clin. 29:317–330
Subhawong TK, Wang KC, Thawait SK et al (2012) High resolution imaging of tunnels by magnetic resonance neurography. Skeletal Radiol 41:15–31. https://doi.org/10.1007/s00256-011-1143-1
Burge AJ, Gold SL, Kuong S, Potter HG (2014) High-resolution magnetic resonance imaging of the lower extremity nerves. Neuroimaging Clin. N. Am.
Chhabra A, Andreisek G, Soldatos T et al (2011) MR neurography: past, present, and future. Am J Roentgenol 197:583–591. https://doi.org/10.2214/AJR.10.6012
Chhabra A (2013) Magnetic resonance neurography-simple guide to performance and interpretation. Semin Roentgenol 48:111–125. https://doi.org/10.1053/j.ro.2012.11.004
Kamath S, Venkatanarasimha N, Walsh MA, Hughes PM (2008) MRI appearance of muscle denervation. Skeletal Radiol. 37:397–404
Aagaard BD, Lazar DA, Lankerovich L et al (2003) High-resolution magnetic resonance imaging is a noninvasive method of observing injury and recovery in the peripheral nervous system. Neurosurgery. https://doi.org/10.1227/01.NEU.0000069534.43067.28
Kwee RM, Chhabra A, Wang KC et al (2014) Accuracy of MRI in diagnosing peripheral nerve disease: a systematic review of the literature. Am J Roentgenol 203:1303–1309. https://doi.org/10.2214/AJR.13.12403
Chhabra A, Flammang A, Padua A et al (2014) Magnetic resonance neurography: technical considerations. Neuroimaging Clin N Am 24:67–78. https://doi.org/10.1016/j.nic.2013.03.032
Hiltunen J, Suortti T, Arvela S et al (2005) Diffusion tensor imaging and tractography of distal peripheral nerves at 3 T. Clin Neurophysiol 116:2315–2323. https://doi.org/10.1016/j.clinph.2005.05.014
Bäumer P, Pham M, Ruetters M et al (2014) Peripheral neuropathy: detection with diffusion-tensor imaging. Radiology 273:185–193. https://doi.org/10.1148/radiol.14132837
Stoll G, Wilder-Smith E, Bendszus M (2013) Imaging of the peripheral nervous system. Handbook of clinical neurology, In, pp 137–153
Bouldin TW, Earnhardt TS, Goines ND (1991) Restoration of blood-nerve barrier in neuropathy is associated with axonal regeneration and remyelination. J Neuropathol Exp Neurol 50:719–728. https://doi.org/10.1097/00005072-199111000-00004
Rossi F, Bignotti B, Bianchi L et al (2020) Radiomics of peripheral nerves MRI in mild carpal and cubital tunnel syndrome. Radiol Medica 125:197–203. https://doi.org/10.1007/s11547-019-01110-z
Tagliafico AS, González RP, Rossi F et al (2020) Peripheral nerves: not only cross-sectional area in the era of radiomics. Semin Musculoskelet Radiol 24:175–180. https://doi.org/10.1055/s-0040-1701629
Maas LC (2003) Diffusion tensor imaging: basic principles and emerging clinical applications. Appl Radiol Online 32(Suppl):S49–S57
Khan KA, Jain SK, Sinha VD, Sinha J (2019) Preoperative diffusion tensor imaging: a landmark modality for predicting the outcome and characterization of supratentorial intra-axial brain tumors. World Neurosurg 124:e540–e551. https://doi.org/10.1016/j.wneu.2018.12.146
Martín Noguerol T, Barousse R, Amrhein TJ et al (2020) Optimizing diffusion-tensor imaging acquisition for spinal cord assessment: physical basis and technical adjustments. Radiographics 40:403–427. https://doi.org/10.1148/rg.2020190058
Martín Noguerol T, Barousse R, Socolovsky M, Luna A (2017) Quantitative magnetic resonance (MR) neurography for evaluation of peripheral nerves and plexus injuries. Quant Imaging Med Surg 7:398–421. https://doi.org/10.21037/qims.2017.08.01
Martín Noguerol T, Barousse R (2020) Update in the evaluation of peripheral nerves by MRI, from morphological to functional neurography. Radiologia 62:90–101. https://doi.org/10.1016/j.rx.2019.06.005
Wang H, Ma J, Zhao L et al (2016) Utility of MRI diffusion tensor imaging in carpal tunnel syndrome: a meta-analysis. Med Sci Monit 22:736–742. https://doi.org/10.12659/msm.895758
Chen Y, Haacke EM, Li J (2019) Peripheral nerve magnetic resonance imaging. F1000Research. https://doi.org/10.12688/f1000research.19695.1
Bruno F, Arrigoni F, Mariani S et al (2019) Application of diffusion tensor imaging (DTI) and MR-tractography in the evaluation of peripheral nerve tumours: State of the art and review of the literature. Acta Biomed. 90:68–76
Chhabra A, Thakkar RS, Andreisek G et al (2013) Anatomic MR imaging and functional diffusion tensor imaging of peripheral nerve tumors and tumorlike conditions. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A3316
Martín Noguerol T, Martínez Barbero JP (2017) RM-Difusión avanzada y biomarcadores en el sistema nervioso central: un nuevo enfoque. Radiologia 59:273–285. https://doi.org/10.1016/j.rx.2017.04.009
Martín-Noguerol T, Montesinos P, Barousse R, Luna A (2021) RadioGraphics update: functional MR neurography in evaluation of peripheral nerve trauma and postsurgical assessment. Radiographics. https://doi.org/10.1148/rg.2021200190
Yao L, Gai N (2009) Median nerve cross-sectional area and MRI diffusion characteristics: normative values at the carpal tunnel. Skeletal Radiol 38:355–361. https://doi.org/10.1007/s00256-008-0626-1
Carro LP, Hernando MF, Cerezal L et al (2016) Deep gluteal space problems: piriformis syndrome, ischiofemoral impingement and sciatic nerve release. Muscles Ligaments Tendons J 6:384–396. https://doi.org/10.11138/mltj/2016.6.3.384
Martín Noguerol T, Barousse R, Gómez Cabrera M, et al (2019) Functional MR neurography in evaluation of peripheral nerve trauma and postsurgical assessment. RadioGraphics 180112. https://doi.org/10.1148/rg.2019180112
Yamasaki T, Fujiwara H, Oda R et al (2015) In vivo evaluation of rabbit sciatic nerve regeneration with diffusion tensor imaging (DTI): correlations with histology and behavior. Magn Reson Imaging 33:95–101. https://doi.org/10.1016/j.mri.2014.09.005
Bikis C, Degrugillier L, Thalmann P et al (2018) Three-dimensional imaging and analysis of entire peripheral nerves after repair and reconstruction. J Neurosci Methods 295. https://doi.org/10.1016/j.jneumeth.2017.11.015
Gallagher TA, Simon NG, Kliot M (2015) Diffusion tensor imaging to visualize axons in the setting of nerve injury and recovery. Neurosurg Focus 39:1–6. https://doi.org/10.3171/2015.6.FOCUS15211
Griffin MF, Malahias M, Hindocha S, Khan WS (2014) Peripheral nerve injury: principles for repair and regeneration. Open Orthop J 8:199–203
Takagi T, Nakamura M, Yamada M et al (2009) Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. Neuroimage 44:884–892. https://doi.org/10.1016/j.neuroimage.2008.09.022
Heckel A, Weiler M, Xia A et al (2015) Peripheral nerve diffusion tensor imaging: assessment of axon and myelin sheath integrity. PLoS One 10:e0130833. https://doi.org/10.1371/journal.pone.0130833
Barcelo C, Faruch M, Lapegue F et al (2013) 3-T MRI with diffusion tensor imaging and tractography of the median nerve. Eur Radiol 23:3124–3130. https://doi.org/10.1007/s00330-013-2955-2
Naraghi A, da Gama LL, Menezes R et al (2013) Diffusion tensor imaging of the median nerve before and after carpal tunnel release in patients with carpal tunnel syndrome: feasibility study. Skeletal Radiol 42:1403–1412. https://doi.org/10.1007/s00256-013-1670-z
Ahlawat S, Chhabra A, Blakely J (2014) Magnetic resonance neurography of peripheral nerve tumors and tumorlike conditions. Neuroimaging Clin N Am 24:171–192. https://doi.org/10.1016/j.nic.2013.03.035
Mazal AT, Ashikyan O, Cheng J et al (2019) Diffusion-weighted imaging and diffusion tensor imaging as adjuncts to conventional MRI for the diagnosis and management of peripheral nerve sheath tumors: current perspectives and future directions. Eur Radiol 29:4123–4132. https://doi.org/10.1007/s00330-018-5838-8
Soldatos T, Fisher S, Karri S et al (2015) Advanced MR imaging of peripheral nerve sheath tumors including diffusion imaging. Semin Musculoskelet Radiol 19:179–190. https://doi.org/10.1055/s-0035-1546823
Cage TA, Yuh EL, Hou SW et al (2015) Visualization of nerve fibers and their relationship to peripheral nerve tumors by diffusion tensor imaging. Neurosurg Focus 39:E16. https://doi.org/10.3171/2015.6.FOCUS15235
Kobayashi S, Hayakawa K, Nakane T et al (2009) Visualization of intraneural edema using gadolinium-enhanced magnetic resonance imaging of carpal tunnel syndrome. J Orthop Sci 14:24–34. https://doi.org/10.1007/s00776-008-1291-x
Lavini C, Buiter MS, Maas M (2013) Use of dynamic contrast enhanced time intensity curve shape analysis in MRI: theory and practice. Reports Med Imaging 6:71–82. https://doi.org/10.2147/RMI.S35088
Hedgire SS, Eberhardt SC, Borczuk R et al (2014) Interpretation and reporting multiparametric prostate MRI: a primer for residents and novices. Abdom Imaging 1–16. https://doi.org/10.1007/s00261-014-0097-x
Ferré J-C, Shiroishi MS, Law M (2012) Advanced techniques using contrast media in neuroimaging. Magn Reson Imaging Clin N Am 20:699–713. https://doi.org/10.1016/j.mric.2012.07.007
Choyke PL, Dwyer AJ, Knopp MV (2003) Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 17:509–520. https://doi.org/10.1002/jmri.10304
Jahng G-H, Li K-L, Ostergaard L, Calamante F (2014) Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol 15:554–577. https://doi.org/10.3348/kjr.2014.15.5.554
Subhawong TK, Wilky BA (2015) Value added: functional MR imaging in management of bone and soft tissue sarcomas. Curr. Opin. Oncol. 27:323–331
Kim SH, Lee HS, Kang BJ et al (2016) Dynamic contrast-enhanced MRI perfusion parameters as imaging biomarkers of angiogenesis. PLoS One 11:e0168632. https://doi.org/10.1371/journal.pone.0168632
Noguerol TM, Luna A, Cabrera MG, Riofrio AD (2017) Clinical applications of advanced magnetic resonance imaging techniques for arthritis evaluation. World J Orthop 8:660–673. https://doi.org/10.5312/wjo.v8.i9.660
Lavini C, Buiter MS, Maas M (2013) Use of dynamic contrast enhanced time intensity curve shape analysis in MRI: theory and practice. Reports Med. Imaging 6:71–82
Lavini C, Pikaart BP, de Jonge MC et al (2009) Region of interest and pixel-by-pixel analysis of dynamic contrast enhanced magnetic resonance imaging parameters and time-intensity curve shapes: a comparison in chondroid tumors. Magn Reson Imaging 27:62–68. https://doi.org/10.1016/j.mri.2008.05.012
Demehri S, Belzberg A, Blakeley J, Fayad LM (2014) Conventional and functional MR imaging of peripheral nerve sheath tumors: initial experience. Am J Neuroradiol 35:1615–1620. https://doi.org/10.3174/ajnr.A3910
Sano Y, Kanda T (2013) Blood-neural barrier: overview and latest progress. Clin Exp Neuroimmunol 4:220–227. https://doi.org/10.1111/cen3.12021
Wasa J, Nishida Y, Tsukushi S et al (2010) MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. Am J Roentgenol 194:1568–1574. https://doi.org/10.2214/AJR.09.2724
Li CS, Huang GS, Da WH et al (2008) Differentiation of soft tissue benign and malignant peripheral nerve sheath tumors with magnetic resonance imaging. Clin Imaging 32:121–127. https://doi.org/10.1016/j.clinimag.2007.05.006
Ahlawat S, Blakeley J, Montgomery E et al (2013) Schwannoma in neurofibromatosis type 1: a pitfall for detecting malignancy by metabolic imaging. Skeletal Radiol. https://doi.org/10.1007/s00256-013-1626-3
Fayad LM, Jacobs MA, Wang X et al (2012) Musculoskeletal tumors: how to use anatomic, functional, and metabolic MR techniques. Radiology
Bäumer P, Reimann M, Decker C et al (2014) Peripheral nerve perfusion by dynamic contrast-enhanced magnetic resonance imaging: demonstration of feasibility. Invest Radiol 49:518–523. https://doi.org/10.1097/RLI.0000000000000046
Rydevik B, Lundborg G (1977) Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scand J Plast Reconstr Surg Hand Surg 11:179–187. https://doi.org/10.3109/02844317709025516
Campagna R, Pessis E, Feydy A et al (2009) MRI assessment of recurrent carpal tunnel syndrome after open surgical release of the median nerve. Am J Roentgenol 193:644–650. https://doi.org/10.2214/AJR.08.1433
Sunderland S (1947) Rate of regeneration of sensory nerve fibers. Arch Neurol Psychiatry 58:1–6. https://doi.org/10.1001/archneurpsyc.1947.02300300011001
Omura K, Ohbayashi M, Sano M et al (2004) The recovery of blood-nerve barrier in crush nerve injury - a quantitative analysis utilizing immunohistochemistry. Brain Res 1001:13–21. https://doi.org/10.1016/j.brainres.2003.10.067
Hill BJ, Padgett KR, Kalra V et al (2018) Gadolinium DTPA enhancement characteristics of the rat sciatic nerve after crush injury at 4.7T. Am J Neuroradiol 39:177–183. https://doi.org/10.3174/ajnr.A5437
Wessig C (2011) Detection of blood-nerve barrier permeability by magnetic resonance imaging. Methods Mol Biol 686:267–271. https://doi.org/10.1007/978-1-60761-938-3_12
Martín Noguerol T, Paulano-Godino F, Martín-Valdivia MT et al (2019) Strengths, weaknesses, opportunities, and threats analysis of artificial intelligence and machine learning applications in radiology. J Am Coll Radiol 16:1239–1247. https://doi.org/10.1016/j.jacr.2019.05.047
Hirschmann A, Cyriac J, Stieltjes B et al (2019) Artificial intelligence in musculoskeletal imaging: review of current literature, challenges, and trends. Semin Musculoskelet Radiol 23:304–311. https://doi.org/10.1055/s-0039-1684024
Shin Y, Yang J, Lee YH, Kim S (2021) Artificial intelligence in musculoskeletal ultrasound imaging. Ultrasonography
Saba L, Biswas M, Kuppili V, et al (2019) The present and future of deep learning in radiology. Eur. J. Radiol.
Zhao H, Sun N (2017) Improved U-net model for nerve segmentation. In: Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics)
Liu C, Liu F, Wang L et al (2018) Segmentation of nerve on ultrasound images using deep adversarial network. Int J Innov Comput Inf Control.
Bowness J, El-Boghdadly K, Burckett-St Laurent D (2020) Artificial intelligence for image interpretation in ultrasound-guided regional anaesthesia. Anaesthesia. https://doi.org/10.1111/anae.15212
Huang C, Zhou Y, Tan W et al (2019) Applying deep learning in recognizing the femoral nerve block region on ultrasound images. Ann Transl Med. https://doi.org/10.21037/atm.2019.08.61
Balsiger F, Steindel C, Arn M et al (2018) Segmentation of peripheral nerves from magnetic resonance neurography: a fully-automatic, deep learning-based approach. Front Neurol 9:1–9. https://doi.org/10.3389/fneur.2018.00777
Haralick RM, Dinstein I, Shanmugam K (1973) Textural features for image classification. IEEE Trans Syst Man Cybern SMC-3:610–621. https://doi.org/10.1109/TSMC.1973.4309314
Tagliafico AS, Tagliafico G (2014) Fascicular ratio: a new parameter to evaluate peripheral nerve pathology on magnetic resonance imaging: a feasibility study on a 3T MRI system. Med (United States) 93. https://doi.org/10.1097/MD.0000000000000068
Tagliafico AS., Rossi F., Valdora F., Grandis M. , Benedetti L., Bignotti B. SA (2019) Feasibility of imaging phenotyping (radiomics) on nuclear MRI of major lower limb nerves. G Ital di Radiol Medica 25–20
Author information
Authors and Affiliations
Contributions
Martín-Noguerol T, designed this work; Martín-Noguerol T, Barousse R and Socolovsky M collected the data, Martín-Noguerol T, Barousse R, Luna A, Socolovsky M, Górriz JM, and Gómez-Río M drafted and critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The author declares a conflict of interest.
Antonio Luna, MD, Ph.D. is occasional lecturer of Philips, Siemens Healthineers, Bracco and Canon and receives royalties as book editor from Springer-Verlag.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The online version of the article contains an error. Figure 4 was accidentally replaced during correction stage.
Rights and permissions
About this article
Cite this article
Martín-Noguerol, T., Barousse, R., Luna, A. et al. New insights into the evaluation of peripheral nerves lesions: a survival guide for beginners. Neuroradiology 64, 875–886 (2022). https://doi.org/10.1007/s00234-022-02916-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-02916-x