Abstract
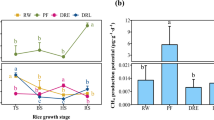
The area of rice paddy fields has declined continuously in East Asian countries due to abandonment of agriculture and concurrent socioeconomic changes. When they are abandoned, rice paddy fields generally transform into wetlands by natural succession. While previous studies have mainly focused on vegetation shifts in abandoned rice paddies, little information is available about how these changes may affect their contribution to wetland functions. As newly abandoned fields proceed through succession, their hydrology and plant communities often change. Moreover, the relationships between these changes, soil microbial characteristics, and emissions of greenhouse gasses are poorly understood. In this study, we examined changes over the course of secondary succession of abandoned rice paddies to wetlands and investigated their ecological functions through changes in greenhouse gas fluxes and microbial characteristics. We collected gas and soil samples in summer and winter from areas dominated by Cyperaceae, Phragmites, and Sphagnum in each site. We found that CO2 emissions in summer were significantly higher than those in winter, but CH4 and N2O emission fluxes were consistently at very low levels and were similar among seasons and locations, due to their low nutrient conditions. These results suggest that microbial activity and abundance increased in summer. Greenhouse gas flux, soil properties, and microbial abundance were not affected by plant species, although the microbial community composition was changed by plant species. This information adds to our basic understanding of the contribution of wetlands that are transformed from abandoned rice paddy systems.






Similar content being viewed by others
References
Maltby E, Holdgate M, Aceman MC, Weir A (1999) Ecosystem management: questions for science and society. Sibthorp Trust, Liverpool
Korea Institute of Geoscience and Mineral Resources. (2012) Searching system of geological information. http://www.kigam.re.kr. Accessed on 1 December 2012
Park MY, Yim YR, Kim HG, Joo YW (2006) The state and characteristics of wetlands created from within abandoned rice paddy fields in South Korea. J Korean Environ Res Reveg Tech 9:1–15
Fukamachi K, Hirokazu O, Nakashizuka T (2001) The change of a satoyama landscape and its causality in Kamiseya, Kyoto Prefecture, Japan between 1970 and 1995. Landsc Ecol 16:703–717
Dennis N (2016) Nature from nature. Science 351(6726):908–910
Drenovsky RE, Vo D, Graham KJ, Scow KM (2004) Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb Ecol 48:424–430
Jaatinen K, Fritze H, Laine J, Laiho R (2007) Effects of short- and long-term water-level drawdown on the populations and activity of aerobic decomposers in a boreal peatland. Glob Chang Biol 13:491–510
Kogi J, Miyamoto M, Bolthouse J, Yokohari M (2010) The potential for abandoned paddy fields to reduce pollution loads from households in suburban Tokyo. Water 2:649–667
Park J, Hong MG, Kim JG (2013) Relationship between early development of plant community and environmental condition in abandoned paddy terraces at mountainous valleys in Korea. J Ecol Environ 36:131–140
Rouhier H, Read DJ (1998) The role of mycorrhiza in determining the response of Plantago lanceolata to CO2 enrichment. New Phytol 139:367–373
Schindlbacher A, Zechmeister-Boltenstern S, Butterbach-Bahl K (2004) Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J Geophys Res 109:1–12
Braker G, Conrad R (2011) Diversity, structure, and size of N2O-producing microbial communities in soils—what matters for their functioning? Adv Appl Microbiol 75:33–70
Butterbach-Bahl K, Dannenmann M (2011) Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr Opin Environ Sustain 3:389–395
Raich JW, Tufekcioglu A (2000) Vegetation and soil respiration: correlations and controls. Biogeochemistry 48:71–79
Hong MG, Kim JG (2013) Inhabitation characteristics of Sphagnum palustre in abandoned paddy terraced wetland: a case report in Ansan. J Wetlands Res 15:71–78
Gelderman RH, Beegle D (1998) Nitrate–nitrogen. Recommended chemical soil test procedures for the North Central Region. North Central Regional Research Publication No. 221 (Revised). Missouri Agricultural Experiment Station, Columbia
Dorich RA, Nelson DW (1983) Direct measurement of ammonium in potassium chloride extracts of soils. Soil Sci Soc Am J 47:833–836
Duchemin E, Lucotte M, Canuel R (1999) Comparison of static chamber and thin boundary layer equation methods for measuring greenhouse gas emissions from large water bodies. Environ Sci Technol 2:350–357
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Rochette P, Gregorich EG, Desjardins RL (1992) Comparison of static and dynamic closed chambers for measurement of soil respiration under field conditions. Can J Soil Sci 72:605–609
Anthony WH, Hutchinson GL, Livingston GP (1995) Chamber measurement of soil–atmosphere gas exchange: linear vs. diffusion-based flux models. Soil Sci Soc Am J 59:1308–1310
Freeman C, Liska G, Ostle NJ, Lock MA, Reynolds B, Hudson J (1996) Microbial activity and enzymic decomposition processes following peatland water table drawdown. Plant Soil 180:121–127
Saiya-Cork R, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, p 115e175
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol Ecol 2:113e118
White T, Bruns T, Lee S, Taylor J, Innis M, Gelfand D, Sninsky J (1990) PCR protocols: a guide to methods and applications. Academic Press, New York
Heid CA, Stevens J, Livak KL, Williams PM (1996) Real time quantitative PCR. Genome Meth 6:986–994
Henry S, Baudoin E, López-Gutiérrez JC, Martin-Laurent F, Brauman A, Philippot L (2004) Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J Microbiol Methods 59:327–335
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene encoding the nitrous oxide reductase and comparison of the abundance of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72(8):5181–5189
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423
Kripalani RH, Kim BJ, Oh JH, Moon SE (2002) Relationship between Soviet snow and Korean rainfall. Int J Climatol 22:1313–1325
Silvola J, Alm J, Ahlholm U, Nykänen H, Martikainen PJ (1996) CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. J Ecol 84:219–228
Forbrich I, Kutzbach L, Hormann A, Wilmking M (2010) A comparison of linear and exponential regression for estimating diffusive CH4 fluxes by closed-chambers in peatlands. Soil Biol Biochem 42:507–515
King GM, Roslev P, Skovgaard H (1990) Distribution and rate of methane oxidation in sediments of the Florida Everglades. Appl Environ Microbiol 56:2902–2911
Bosse U, Frenzel P, Conrad R (1993) Inhibition of methane oxidation by ammonium in the surface layer of a littoral sediment. FEMS Microbiol Ecol 13:123–134
Haichar FZ, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J (2008) Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2:1221–1230
Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N et al (2010) Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329:834–838
Li C, Qiu J, Frolking S, Xiao X, Salas W, Moore B, Boles S, Huang Y, Sass R (2002) Reduced methane emissions from large-scale changes in water management of China’s rice paddies during 1980–2000. Geophys Res Lett 29(20):1972
Jassey VE, Chiapusio G, Binet P, Buttler A, Laggoun F, Delarue F, Bernard N, Mitchell EA, Toussaint M, Francez A, Gilbert D (2013) Above- and belowground linkages in Sphagnum peatland: climate warming affects plant-microbial interactions. Glob Change Biol 19:811–823
Hemond H (1983) The nitrogen budget of Thoreaus bog. Ecology 64:99–109
Kazuyuki I, Cheng W, Aonuma S, Hoque MM, Kobayashi K, Miura S, Kim HY, Okada M (2003) Effects of free-air CO2 enrichment (FACE) on CH4 emission from a rice paddy field. Glob Chang Biol 9:1458–1464
Martikainen PJ, Nykänen H, Alm J, Silvola J (1995) Change in fluxes of carbon dioxide, methane and nitrous oxide due to forest drainage of mire sites of different trophy. Plant Soil 68–169:571–577
Martikainen PJ, Nykänen H, Crill P, Silvola J (1993) Effect of a lowered water table on nitrous oxide fluxes from northern peatlands. Nature 366:51–53
Seitzinger SP (1994) Linkages between organic matter mineralization and denitrification in eight riparian wetlands. Biogeochemistry 25:19–39
Bowden WB, McDowell WH, Asbury CE, Finley AM (1992) Riparian nitrogen dynamics in two geomorphologically distinct tropical rain forest watersheds: nitrous oxide fluxes. Biogeochemistry 18:77–99
Sugiyama S, Zabed HM, Okubo A (2008) Relationships between soil microbial diversity and plant community structure in semi-natural grasslands. Grassland Sci 54:117–124
Suseela V, Conant RT, Wallenstein MD, Dukes JS (2012) Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob Chang Biol 18:336–348
Bajracharya RM, Lal R, Kimble JM (2000) Erosion effects on carbon dioxide concentration and carbon flux from an Ohio alfisol. Soil Sci Soc Am J 64:694
Bajracharya RM, Lal R, Kimble JM (2000) Diurnal and seasonal CO2-C flux from soil as related to erosion phases in central Ohio. Soil Sci Soc Am J 64:286–293
Follett RF (1997) CRP and microbial biomass dynamics in temperate climates. In: Lal R (ed) Management of carbon sequestration in soil. CRC Press, Boca Raton, pp 305–322
Ding W, Cai Z, Tsuruta H (2005) Plant species effects on methane emissions from freshwater marshes. Atmos Environ 39:3199–3207
Shin YK, Lee YS, Yun SH (1996) Methane emission measurement in rice paddy of Korea. In: Environmental and biometeorology—the Proceedings of International Symposium on Environment and Biometeorology. Agricultural Scientech Press, Beijing, pp 495–503
Shin YK, Yun SH (2000) Varietal differences in methane emission from Korean rice cultivars. Nutr Cycl Agroecosyst 58:315–319
Ko JY, Kang HW, Kang UG (1998) The effects of nitrogen fertilizers and cultural patterns on methane emission from rice paddy fields. Korean J Environ Agric 17:227–233 (in Korean)
Ko JY, Kang HW, Park KB (1996) Effects of water management rice straw and compost on methane emission in dry seeded rice. J Korean Soc Soil Sci Fertilizer 29:212–217 (in Korean)
Lee KB, Lee DB, Kim JG (2000) Effects of application of nitrogen fertilizers on methane emission in a paddy soil. J Korean Soc Soil Sci Fertilizer 33:212–219 (in Korean)
Lee KB, Lee DB, Lee SB (1999) Methane emission among rice ecotypes in Korean paddy soil. Korean J Environ Agric 18:1–5
Lee KB, Lee DB, Kim JG (1997) Effect of rice cultural patterns on methane emission from a Korean paddy soil. J Korean Soc Soil Sci Fertilizer 30:35–40 (in Korean)
Lai DYF (2009) Methane dynamics in northern peatlands: a review. Pedosphere 19:409–421
Morse JL, Ardón M, Bernhardt ES (2012) Greenhouse gas fluxes in southeastern U.S. coastal plain wetlands under contrasting land uses. Ecol Appl 22:264–280
Takai K, Horikoshi K (2000) Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol 66:5066–5072
Liu X, Tiquia SM, Holguin G, Wu L, Nold SC, Devol AH, Luo K, Palumbo AV, Tiedje JM, Zhou J (2003) Molecular diversity of denitrifying genes in continental margin sediments within the oxygen-deficient zone off the Pacific Coast of Mexico. Appl Environ Microbiol 69:3249–3560
Luesters T, Friedrich NW (2003) Evaluation of PCR amplification bias by terminal restriction fragment length polymorphism analysis of small-subunit rRNA and mcrA genes by using defined template mixtures of methanogenic pure cultures and soil DNA extracts. Appl Environ Microbiol 69:32–326
Holmes AJ, Costello A, Lidstrom ME, Murrell JC (1995) Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett 132:203–208
Acknowledgments
This study was supported by ERC (No. 2011-0030040). S. Kim was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (86457858).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1
Primer for T-RFLP and quantitative PCR used in this study (DOCX 17.5 KB)
Table 2
Annual growth in length, and dry mass of plants in each sites (DOCX 11 kb)
Table 3
Shannon-Weaver index of diversity (H’) determined based on T-RFLP analysis. (DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Kim, S., Lee, S., McCormick, M. et al. Microbial Community and Greenhouse Gas Fluxes from Abandoned Rice Paddies with Different Vegetation. Microb Ecol 72, 692–703 (2016). https://doi.org/10.1007/s00248-016-0801-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0801-1