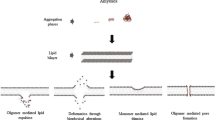
Abstract
The fate of proteins with amyloidogenic properties depends critically on their immediate biochemical environment. However, the role of biological interfaces such as membrane surfaces, as promoters of pathological aggregation of amyloidogenic proteins, is rarely studied and only established for the amyloid-β protein (Aβ) involved in Alzheimer’s disease, and α-synuclein in Parkinsonism. The occurrence of binding and misfolding of these proteins on membrane surfaces, is poorly understood, not at least due to the two-dimensional character of this event. Clearly, the nature of the folding pathway for Aβ protein adsorbed upon two-dimensional aggregation templates, must be fundamentally different from the three-dimensional situation in solution. Here, we summarize the current research and focus on the function of membrane interfaces as aggregation templates for amyloidogenic proteins (and even prionic ones). One major aspect will be the relationship between membrane properties and protein association and the consequences for amyloidogenic products. The other focus will be on a general understanding of protein folding pathways on two-dimensional templates on a molecular level. Finally, we will demonstrate the potential importance of membrane-mediated aggregation for non-amphiphatic soluble amyloidogenic proteins, by using the SOD1 protein involved in the amyotrophic lateral sclerosis syndrome.






Similar content being viewed by others
References
Balakrishnan S, Goodwin H, Cumings JN (1961) The distribution of phosphorus-containing lipid compounds in the human brain. J Neurochem 8:276–284
Barnham KJ, Ciccotosto GD, Tickler AK, Ali FE, Smith DG, Williamson NA, Lam Y-H, Carrington D, Tew D, Kocak G, Volitakis I, Separovic F, Barrow CJ, Wade JD, Masters CL, Cherny RA, Curtain CC, Bush AI, Cappai R (2003) Neurotoxic, redox-competent Alzheimer’s β amyloid is released from lipid membrane by methionine oxidation. J Biol Chem 278:42959–42965
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:205–214
Barnham KJ, Cappai R, Beyreuther K, Masters CL, Hill AF (2006) Delineating common molecular mechanisms in Alzheimer’s and prion diseases. Trends Biochem Sci 31:465–472
Bokvist M, Lindström F, Watts A, Gröbner G (2004) Two types of Alzheimer’s β-amyloid (1–40) peptide membrane interactions: aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J Mol Biol 335:1039–1049
Bonev BB, Chan WC, Bycroft BW, Roberts GCK, Watts A (2000) Interaction of the lantibiotic nisin with mixed lipid bilayers: a 31P and 2H NMR study. Biochemistry 39:11425–11433
Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW (1998) Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 281:1851–1854
Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M (2002) Inherent toxicity of aggregates implies a common mechanism for peptide misfolding diseases. Nature 416:507–511
Carbone MA, Macdonald PM (1996) Cardiotoxin II segregates phosphatidylglycerol from mixtures with phosphatidylcholine: P-31 and H-2 NMR spectroscopic evidence. Biochemistry 35:3368–3378
Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI (2001) Treatment with a copper–zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30:665–676
Curtain CC, Ali FE, Smith DG, Bush AI, Masters CL, Barnham KJ (2003) Metal ions, pH, and cholesterol regulate the interactions of Alzheimer’s disease amyloid-β peptide with membrane lipid. J Biol Chem 278:2977–2982
Dai XL, Sun YX, Jiang ZF (2006) Cu (II) potentiation of Alzheimer Abeta 1–40. cytotoxicity and transition on its secondary structure. Acta Biochim Biophys Sin (Shanghai) 38:765–772
Danielsson J, Pierattelli R, Banci L, Gräslund A (2007) High-resolution NMR studies of the zinc-binding site of the Alzheimer’s amyloid β-peptide. FEBS J 274:46–59
Demmester N, Baier G, Enzinger C, Goethals M, Vandekerckhove J, Rosseneu M, Labeur C (2000) Apoptosis induced in neuronal cells by C-terminal amyloid β-fragments is correlated with their aggregation properties in phospholipid membranes. Mol Membr Biol 17:219–228
Devanathan S, Salamon Z, Lindblom G, Gröbner G, Tollin G (2006) Effects of sphingomyelin, cholesterol and zinc ions on the binding, insertion and oligomerization of the amyloid Aβ1–42 peptide in solid-supported lipid bilayers. FEBS J 273:1389–1402
Durell SR, Guy HR, Arispe N, Rojas E, Pollard HB (1994) Theoretical models of the ion channel structure of amyloid β-protein. Biophys J 67:2137–2145
Ege C, Mayewski WG, Kjaer K, Lee KYC (2005) Templating effect of lipid membranes on Alzheimer’s amyloid beta peptide. Chem Phys Chem 6:226–229
Fernandez A, Berry RS (2003) Proteins with H-bond packing defects are highly interactive with lipid bilayers: implications for amyloidogenesis. Proc Natl Acad Sci USA 100:2391–2396
Fernandez A, Kardos J, Scott LR, Goto Y, Berry RS (2003) Structural defects and the diagnosis of amyloidogenic propensity. Proc Natl Acad Sci USA 100:6446–6451
Giacomelli CE, Norde W (2005) Conformational changes of the amyloid β-peptide (1–40) adsorbed on solid surfaces. Macromol Biosci 5:401–407
Gibson Wood W, Eckert GP, Igbavboa U, Müller WE (2003) Amyloid beta-protein interactions with membranes and cholesterol: causes or casualties of Alzheimer’s disease. Biochim Biophys Acta 1610:281–290
Glabe CG (2006) Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neuro-biology of aging 4:570–575
Grimm MOW, Grimm HS, Pätzold AJ, Zinser EG, Halonen R, Duering M, Tschäpe J-A, De Strooper B, Mueller U, Shen J, Hartmann T (2005) Regulation of cholesterol and sphingomyelin metabolism by amyloid-β and presenilin. Nature Cell Biol 11:1118–1123
Haass C, Selkoe DJ (1993) Cellular processing of β-amyloid precursor peptide and the genesis of amyloid beta-peptide. Cell 75:1039–1042
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Mol Cell Biol 8:101–112
Iversen LL, Mortishire-Smith RJ, Pollack SJ, Shearman MS (1995) The toxicity in-vitro of β-amyloid peptide. Biochem J 311:1–16
Jimenez JL, Guijarro JI, Orlova E, Zurdo J, Dobson CM, Sunde M, Saibil HR (1999) Cryo-electron microscopy structure of an SH3 amyloid fibril and model of the molecular packing. EMBO J 18:815–821
Kakio A, Nishimoto S, Yanagisawa K, Kozutsumi Y, Matsuzaki K (2002) Interactions of amyloid β-peptide with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry 41:7385–7390
Kawahara M, Kuroda Y, Arispe N, Rojas E (2000) Alzheimer’s β-amyloid, human islet amylin, and prion peptide fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J Biol Chem 275:14077–14083
Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe C (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300:486–489
Klein WL, Stine WB Jr, Teplow DB (2004) Small assemblies of unmodified amyloid-β-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol Aging 25:569–580
Kong GK-W, Adams JJ, Harris HH, Boas JF, Curtain CC, Galatis D, Masters CL, Barnham KJ, McKinstry WJ, Cappai R, Parker MW (2007) Structural studies of the Alzheimer’s amyloid precursor protein copper-binding domain reveal how it binds copper ions. J Mol Biol 367:148–161
Kuchinka E, Seelig J (1989) Interaction of melittin with phosphatidylcholine membranes: binding isotherm and lipid headgroup conformation. Biochemistry 28:4216–4221
Lansbury PT Jr (1999) Evolution of amyloid: what normal peptide folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci USA 96:3342–3344
Lau TL, Ambroggio EE, Tew JD, Cappai R, Masters CL, Fidelio GD, Barnham KJ, Separovic F (2006) Amyloid-β peptide disruption of lipid membranes and the effect of metal ions. J Mol Biol 356:759–770
Lin H, Bhatia R, Lal R (2001) Amyloid-β protein forms ion channels: implications for Alzheimer’s disease pathology. FASEB J 15:2433–2444
Lindberg MJ, Bystrom R, Boknas N, Andersen PM, Oliveberg M (2005). Systematically perturbed folding patterns of amyotrophic lateral sclerosis (ALS)-associated SOD1 mutants. Proc Natl Acad Sci USA 102:9754–9759
Lindström F, Bokvist M, Sparrman T, Gröbner G (2002) Association of amyloid-β peptide with membrane surfaces monitored by solid state NMR. Phys Chem Chem Phys 4:5524–5530
Lindström F, Williamson PTF, Gröbner G (2005) Molecular insight into the electrostatic membrane surface potential by 14N/31P MAS NMR spectroscopy: nociceptin–lipid association. J Am Chem Soc 127:6610–6616
Liu J, Lillo C, Jonsson PA, Velde CV, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, Brännström T, Gredal O, Wong PC, Williams DS, Cleveland DW (2004) Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron 43:5–17
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and down syndrome. Proc Natl Acad Sci USA 82:4245–4249
McLaurin J, Yang D-S, Yip CM, Fraser PE (2000) Review: modulating factors in amyloid-β fibril formation. J Struct Biol 130:259–270
Minton AP (1999) Adsorption of globular proteins on locally planar surfaces. II. models for the effect of multiple adsorpate conformations on adsorption equilibria and kinetics. Biophys J 76:176–187
Pinheiro TJT, Watts A (1994) Resolution of individual lipids in mixed phospholipid-membranes and specific lipid cytochrome-C interactions by magic-angle-spinning solid-state P-31 NMR. Biochemistry 33:2459–2467
Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL (2003) Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting A beta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 60:1685–1691
Rochet J-C, Lansbury PT Jr (2000) Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol 10:60–68
Rochet J-C, Outeiro TF, Conway KA, Ding TT, Volles MJ, Lashuel HA, Bieganski RM, Lindquist SL, Lansbury PT (2004) Interactions among alpha-synuclein, dopamine, and biomembranes: some clues for understanding neurodegeneration in Parkinson’s disease. J Mol Neurosci 23:23–33
Scheuermann S, Hambsch B, Hesse L, Stumm J, Schmidt C, Beher D, Bayer TA Beyreuther K, Multhaup G (2001) Homodimerization of amyloid precursor protein and its implication in the amyloidogenic pathway of Alzheimer’s disease. J Biol Chem 276:33923–33929
Seelig J (1978) 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta 515:105–140
Selkoe DJ (2004) Review: cell biology of misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol 6:1054–1061
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007) Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss of modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27:2866–2875
Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K, (1998) Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 95:6460–6464
Smith DP, Smith DG, Curtain CC, Boas JF, Pilbrow JR, Ciccotosto GD, Lau T-L, Tew DJ, Perez K, Wade JD, Bush AI, Drew SC, Separovic F, Masters CL, Cappai R, Barnham KJ (2006) Copper-mediated amyloid-β toxicity is associated with an intermolecular histidine bridge. J Biol Chem 281:15145–15154
Smith DP, Ciccotosto GD, Tew DJ, Fodero-Tavoletti MT, Johanssen T, Masters CL, Barnham KJ, Cappai R (2007) Concentration dependent Cu2+ induced aggregation and dityrosine formation of the Alzheimer’s disease amyloid-β peptide. Biochemistry 46:2881–2891
Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P (2005) Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci 48:1051–158
Sparr E, Engel M, Sakharov D, Sprong M, Jacobs J, de Kruijff B, Höppener J, Killian JA (2004) Islet amyloid polypeptide-induced membrane leakage involves uptake of lipids by forming amyloid fibers. FEBS Lett 577:117–120
Terzi E, Hölzemann G, Seelig A (1995) Self-association of beta-amyloid peptide (1–40) in solution and binding to lipid-membranes. J Mol Biol 252:633–642
Terzi E, Hölzemann G, Seelig J (1997) Interaction of Alzheimer β-amyloid peptide (1–40) with lipid membranes. Biochemistry 36:14845–14852
Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB (1999) Amyloid β-peptide fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem 274:25945–25952
Walsh D, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ (2000) The oligomerization of amyloid β-peptide begins intracellularly in cells derived from human brain. Biochemistry 39:10831–10839
Waschuk SA, Elton EA, Darabie AA, Fraser PE, McLaurin J (2001) Cellular membrane composition defines Aβ-lipid interactions. J Biol Chem 276:33561–33568
Wieprecht T, Apostolov O, Beyermann M, Seelig J (2000) Membrane binding and pore formation of the antibacterial peptide PGLa: thermodynamic and mechanistic aspects. Biochemistry 2000:442–452
Zetterström P, Stewart HG, Bergemalm D, Jonsson PA, Graffmo KS, Andersen PM, Brännström T, Oliveberg M, Marklund SL (2007) Soluble misfolded subfractions of mutant superoxide dismutase-1s are enriched in spinal cords throughout life in murine ALS models. Proc Natl Acad Sci USA 104:104–135
Zhao H, Tuominen EKJ, Kinnunen PKJ (2004) Formation of amyloid fibers triggered by phosphatidylserine-containing membranes. Biochemistry 43:10302–10307
Acknowledgments
This work was supported by the Knut and Alice Wallenberg Foundation, Swedish Research Council (NT and Medicine), Umeå University Biotechnology Fund, Natural Science Faculty and Alzheimer Foundation. We thank M. Oliveberg, S. Marklund, G. Lindblom, L. Johansson and E. Rosenbaum for all their support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Australian Society for Biophysics Special Issue: Metals and Membranes in Neuroscience.
Rights and permissions
About this article
Cite this article
Aisenbrey, C., Borowik, T., Byström, R. et al. How is protein aggregation in amyloidogenic diseases modulated by biological membranes?. Eur Biophys J 37, 247–255 (2008). https://doi.org/10.1007/s00249-007-0237-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-007-0237-0