Abstract
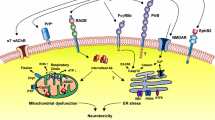
Alzheimer’s disease is the most common form of dementia in the elderly, and is characterised by extracellular amyloid plaques composed of the β-amyloid peptide (Aβ). However, disease progression has been shown to correlate more closely with the level of soluble Aβ oligomers. Recent evidence suggests that these oligomers are covalently crosslinked, possibly due to the interaction of Aβ with redox-active metal ions. These findings offer new avenues for the treatment and prevention of disease, by modulating metal binding or preventing the formation of neurotoxic Aβ oligomers.



Similar content being viewed by others
References
Atwood CS, Perry G, Zeng H, Kato Y, Jones WD, Ling K, Huang X, Moir RD, Wong D, Sayre LM, Smith MA, Chen SG, Bush AI (2004) Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-ß. Biochemistry 43:560–568
Atwood CS, Scarpa RC, Huang X, Moir RD, Jones WD, Fairlie DP, Tanzi RE, Bush AI (2000) Characterization of copper interactions with alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta1–42. J Neurochem 75:1219–1233
Barnham KJ, Haeffner F, Ciccotosto GD, Curtain CC, Tew D, Mavros C, Beyreuther K, Carrington D, Masters CL, Cherny RA, Cappai R, Bush AI (2004) Tyrosine gated electron transfer is key to the toxic mechanism of Alzheimer’s disease beta-amyloid. FASEB J 18:1427–1429
Bitan G, Tarus B, Vollers SS, Lashuel HA, Condron MM, Straub JE, Teplow DB (2003) A molecular switch in amyloid assembly: Met35 and amyloid beta-protein oligomerisation. J Am Chem Soc 125:15359–15365
Cherny R, Legg JT, McLean CA, Fairlie DP, Huang X, Atwood CS, Beyreuther K, Tanzi RE, Masters CL, Bush AI (1999) Aqueous dissolution of Alzheimer’s disease Abeta amyloid depositis by biometal depletion. J Biol Chem 274:232223–232228
Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI (2001) Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30:665–676
Ciccotosto GD, Tew D, Curtain CC, Smith D, Carrington D, Masters CL, Bush AI, Cherny RA, Cappai R, Barnham KJ (2004) Enhanced toxicity and cellular binding of a modified amyloid b peptide with a methionine to valine substitution. J Biol Chem 279:42528–42534
Citron BA, Suo Z, SantaCruz K, Davies PJA, Qin F, Festoff BW (2002) Protein crosslinking, tissue transglutaminase, alternative splicing and neurodegeneration. Neurochem Int 40:69–78
Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A (2006) Opposing activities protect against age-onset proteotoxicity. Science 313:1604–1610
Deshpande A, Mina E, Glabe CG, Busciglio J (2006) Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci 26:6011–6018
Harigaya Y, Saido TC, Eckman CB, Prada CM, Shoji M, Younkin SG (2000) Amyloid beta protein starting pyroglutamate at position 3 is a major component of the amyloid deposits in the Alzheimer’s disease brain. Biochem Biophys Res Comm 276:422–427
Hensley K, Maidt ML, Yu Z, Sang H, Markesbery WR, Floyd RA (1998) Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J Neurosci 18:8126–8132
Ho GJ, Gregory EJ, Smirnova IV, Zoubine MN, Festoff BW (1994) Cross-linking of beta-amyloid precursor catalyzed by tissue transglutaminase. FEBS Lett 349:151–154
Lambert MP, Barlow AK, Chromy BA, Edwards C, Fred R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible, nonfibrillar ligands derived from Ab1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95:6448–6453
Lee H, Zhu X, Castellani RJ, Nunomura A, Perry G, Smith MA (2007) Amyloid-beta in Alzheimer disease: the null versus the alternate hypothesis. J Pharmacol Exp Ther 321:823–9
Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH 2006) A specific amylid-beta protein assembly in the brain impairs memory. Nature 440:352–357
Maurer K, Hoyer S (2006) Alois Alzheimer revisited: differences in origin of the disease carrying his name. J Neural Transm 113:1645–1658
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL (1999) Soluble Pool of Aß Amyloid as a Determinant of Severity of Neurodegeneration in Alzheimer’s Disease. Ann Neurol 46:860–866
Nemes Z, Fesus L, Egerhazi A, Keszthelyi A, Degrell IM (2001) N(epsilon)(gamma-glutamyl)lysine in cerebrospinal fluid marks Alzheimer type and vascular dementia. Neurobiol Aging 22:403–406
Okeley NM, van der Donk WA (2000) Novel cofactors via post-translational modifications of enzyme active sites. Chem Biol 7:R159–171
Petkova AT, Leapman RD, Guo Z, Yau W, Mattson MP, Tycko R (2005) Self-propagating, molecular-level polymorphism in Alzheimer’s ß-amyloid fibrils. Science 307:262–265
Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ (1995) Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem 270:9564–9570
Small DH (2000) Recent findings on the biology of Alzheimer’s disease. Res Pract Alzheimers Dis 3:27–33
Smith DP, Smith DG, Curtain CC, Boas JF, Pilbrow JR, Ciccotosto GD, Lau TL, Tew DJ, Perez K, Wade JD, Bush AI, Drew SC, Separovic F, Masters CL, Cappai R, Barnham KJ (2006) Copper-mediated amyloid-beta toxicity is associated with an intermolecular histidine bridge. J Biol Chem 281:15145–15154
Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ (2006) Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol 572:477–492
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturaly secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416:535–539
Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, El Agnaf O, Hartley DM, Selkoe DJ (2005) Certain inhibitors of synthetic amyloid beta-peptide (Ab) fibrillogenesis block oligomerization of natural Ab and thereby rescue long-term potentiation. J Neurosci 25:2455–2462
Webber KM, Casadesus G, Atwood CS, Bowen RL, Perry G, Smith MA (2007) Gonadotropins: a cohesive gender-based etiology of Alzheimer disease. Mol Cell Endo 260–262:271–275
Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y (2006) Amyloid beta-induced pathological behaviors are suppressed by Gingko biloba extract EGb 761 and ginkolides in transgenic Caenorhabditis elegans. J Neurosci 26:13102–13113
Author information
Authors and Affiliations
Corresponding author
Additional information
Australian Society for Biophysics Special Issue: Metals and Membranes in Neuroscience.
Rights and permissions
About this article
Cite this article
Naylor, R., Hill, A.F. & Barnham, K.J. Neurotoxicity in Alzheimer’s disease: is covalently crosslinked Aβ responsible?. Eur Biophys J 37, 265–268 (2008). https://doi.org/10.1007/s00249-007-0243-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-007-0243-2