Abstract
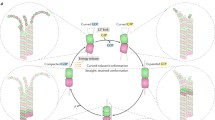
Microtubules have been in the focus of biophysical research for several decades. However, the confusing and mutually contradictory results regarding their elasticity and fluctuations have cast doubt on their present understanding. In this paper, we present the empirical evidence for the existence of discrete guanosine diphosphate (GDP)–tubulin fluctuations between a curved and a straight configuration at room temperature as well as for conformational tubulin cooperativity. Guided by a number of experimental findings, we build the case for a novel microtubule model, with the principal result that microtubules can spontaneously form micron-sized cooperative helical states with unique elastic and dynamic features. The polymorphic dynamics of the microtubule lattice resulting from the tubulin bistability quantitatively explains several experimental puzzles, including anomalous scaling of dynamic fluctuations of grafted microtubules, their apparent length–stiffness relation, and their remarkable curved–helical appearance in general. We point out that the multistability and cooperative switching of tubulin dimers could participate in important cellular processes, and could in particular lead to efficient mechanochemical signaling along single microtubules.












Similar content being viewed by others
Notes
In Appendix E we discuss possible effects induced by surface attachment that could to some extent interfere with the ideal free “wobbling” motion in experiments.
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, 4th edn. Garland Science, New York
Amos LA (1991) Negative Stain electron microscopy of microtubules and associated motor molecules. Micron Microsc Acta 22:395
Amos LA, Amos WG (1991) Molecules of the cytoskeleton. Guilford, New York
Amos LA, Amos WB (1991) The bending of sliding microtubules imaged by confocal light microscopy and negative stain electron microscopy. J Cell Sci Suppl 14:95–101
Amos LA, Löwe J (1999) How taxol stabilises microtubule structure. Chem Biol 6:R65–R69
Arnal I, Wade RH (1995) How does taxol stabilize microtubules?. Curr Biol 5:900–908
Asakura S (1970) Polymerization of flagellin and polymorphism of flagella. Adv Biophys (Japan) 1:99–155
Bicek AD, Tüzel E, Demtchouk A, Uppalapati M, Hancock WO, Kroll DM, Odde DJ (2009) Anterograde microtubule transport drives microtubule bending in LLC-PK1 epithelial cells. Mol Biol Cell 20:2943
Bouchet-Marquis C, Zuber B, Glynn A-M, Eltsov M, Grabenbauer M, Goldie KN, Thomas D, Frangakis AS, Dubochet J, Chrétien D (2007) Visualization of cell microtubules in their native state. Biol Cell 99:45–53
Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, Parker KK, Ingber DE, Weitz DA (2006) Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol 173:733–741
Brangwynne C, Koenderink G, Barry E, Dogic Z, MacKintosh F, Weitz D (2007) Bending dynamics of fluctuating biopolymers probed by automated high-resolution filament tracking. Biophys J 93:346–359
Calladine CR (1975) Construction of bacterial flagella. Nature (London) 255:121–124
Chretien D, Fuller SD (2000) Microtubules switch occasionally into unfavorable configurations during elongation. J Mol Biol 298:663–676
Chretien D, Wade RH (1991) New data on the microtubule surface lattice. Biol Cell 71:161–174
Chrétien D, Metoz F, Verde F, Karsenti E, Wade RH (1992) Lattice defects in microtubules: protofilament numbers vary within individual microtubules. J Cell Biol 117:1031–1040
Chrétien D, Flyvbjerg H, Fuller SD (1998) Limited flexibility of the inter-protofilament bonds in microtubules assembled from pure tubulin. Eur Biophys J 27:490–500
Davis LJ, Odde DJ, Block SM, Gross SP (2002) The importance of lattice defects in Katanin-mediated microtubule severing in vitro. Biophys J 82:2916
Desai A, Verma S, Mitchison TJ, Walczak CE (1999) Kin I kinesins are microtubule-destabilizing enzymes. Cell 96:69–78
Elie-Caille C, Severin F, Helenius J, Howard J, Muller DJ, Hyman AA (2007) Straight GDP–tubulin protofilaments form in the presence of taxol. Curr Biol 17:1765–1770
Erickson HP, O’Brien ET (1992) Microtubule dynamic instability and GTP hydrolysis. Annu Rev Biophys Biomol Struct 21:145–166
Felgner H, Frank R, Schliwa M (1996) Flexural rigidity of microtubules measured with the use of optical tweezers. J Cell Sci 109:509–516
Fridoon JA, Hughey J, Wittmann T, Hyman A, Greaser M, Baas PW (2000) Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nature Cell Biol 2:276
Gittes F, Mickey B, Nettleton J, Howard J (1993) Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol 120:923–934
Hartman JJ, Mahr J, McNally K, Okawa K, Iwamatsu A, Thomas S, Cheesman S, Heuser J, Vale RD, McNally FJ (1998) Katanin, a microtubule-severing protein is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell 93:277–287
Heussinger C, Bathe M, Frey E (2007) Statistical mechanics of semiflexible bundles of wormlike polymer chains. Phys Rev Lett 99:048101
Howard J (2001) Mechanics of motor proteins and the cytoskeleton. Sinauer, Sunderland
Huilin J, De Rosier DJ, Nicholson WV, Nogales E, Downing KH (2002) Microtubule structure at 8 Å resolution. Structure 10:1317–1328
Hunyadi V, Janosi IM (2007) Metastability of microtubules induced by competing internal forces. Biophys J 92:3092–3097
Janosi IM, Chretien D, Flyvbjerg H (2002) Structural microtubule cap: stability, catastrophe, rescue, and third state. Biophys J 83:1317–1330
Janson ME, Dogterom M (2004) A bending mode analysis for growing microtubules: evidence for a velocity-dependent rigidity. Biophys J 87:2723–2736
Kaech S, Ludin B, Matus A (1996) Cytoskeletal plasticity in cells expressing neuronal microtubule-associated proteins. Neuron 17:1189
Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG (1997) Microtubule release from the centrosome. Proc Natl Acad Sci USA 94:5078
Keller PJ, Pampaloni F, Lattanzi G, Stelzer EHK (2008) Three-dimensional microtubule behavior in Xenopus egg extracts reveals four dynamic states and state-dependent elastic properties. Biophys J 95:1474
Kikumoto M, Kurachi M, Tosa V, Tashiro H (2006) Flexural rigidity of individual microtubules measured by a buckling force with optical traps. Biophys J 90:1687–1696
Kim T, Kao MT, Hasselbrink EF, Meyhöfer E (2008) Nanomechanical model of microtubule translocation in the presence of electric fields. Biophys J 94:3880–3892
Kis A, Kasas S, Babic B, Kulik AJ, Benoit W, Briggs GA, Schonenberger C, Catsicas S, Forro L (2002) Nanomechanics of microtubules. Phys Rev Lett 89:248101
Kol N, Adler-Abramovich L, Barlam D, Shneck RZ, Gazit E, Rousso I (2005) Self-assembled peptide nanotubes are uniquely rigid bioinspired supramolecular structures. Nano Lett 5:1343
Kulic IM, Brown AEX, Kim H, Kural C, Blehm B, Selvin PR (2008) The role of microtubule movement in bidirectional organelle transport. Proc Natl Acad Sci USA 105:10011–10016
Kurachi M, Hoshi M, Tashiro H (1995) Buckling of a single microtubule by optical trapping forces: direct measurement of microtubule rigidity. Cell Motil Cytoskeleton 30:221–228
Löwel J, Li H, Downing KH, Nogales E (2001) Refined structure of αβ-Tubulin at 3.5 Å resolution. J Mol Biol 313:10451–10457
Mandelkow EM, Mandelkow E, Milligan RA (1991) Microtubules dynamics and microtubules caps: a time-resolved cryoelectron microscopy study. J Cell Biol 114:977–991
Mickey B, Howard J (1995) Rigidity of microtubules is increased by stabilizing agents. J Cell Biol 130:909–917
Mitchison T, Kirschner MW (1984) Dynamic instability of microtubule growth. Nature 312:237–242
Mohrbach H, Kulic IM (2007) Motor driven microtubule shape fluctuations: force from within the lattice. Phys Rev Lett 99:218102
Mohrbach H, Johner A, Kulic IM (2010) Tubulin bistability and polymorphic dynamics of microtubules. Phys Rev Lett 105:268102
Muto E, Sakai H, Kaseda K (2005) Long-range cooperative binding of kinesin to a microtubule in the presence of ATP. J Cell Biol 168:691
Nogales E, Wolf SG, Downing KH (1998) Structure of the αβ tubulin dimer by electron crystallography. Nature 391:199–203
Nogales E, Whittaker M, Milligan RA, Downing KH (1999) High resolution model of the microtubule. Cell 96:79–88
Nogales E, Wang HW, Niederstrasser H (2003) Tubulin rings: which way do they curve ?. Curr Opin Struct Biol 13:256–261
Pampaloni F, Lattanzi G, Jonas A, Surrey T, Frey E, Florin EL (2006) Thermal fluctuations of grafted microtubules provide evidence of a length-dependent persistence length. Proc Natl Acad USA 103:10248–10253
Ray S, Meyhofer E, Milligan RA, Howard J (1993) Kinesin follows the microtubule’s protofilament axis. J Cell Biol 121:1083–1093
Samsonov A, Yu J-Z, Rasenick M, Popov SV (2004) Tau interaction with microtubules in vivo. J Cell Sci 117:6129
Takasone T, Juodkazis S, Kawagishi Y, Yamaguchi A, Matsuo S, Sakakibara H, Nakayama H, Misawa H (2002) Flexural rigidity of a single microtubule. Jpn J Appl Phys 41:3015–3019
Taute KM, Pampaloni F, Frey E, Florin E-L (2008) Microtubule dynamics depart from the wormlike chain model. Phys Rev Lett 100:028102
Vale RD, Coppin CM, Malik F, Kull FJ, Milligan RD (1994) Tubulin GTP hydrolysis influences the structure, mechanical properties and kinesin-driven transport of microtubules. J Biol Chem 269:23769–23775
Vanden Heuvel MGL, Bolhuis S, Dekker C (2007) Persistence length measurements from stochastic single-microtubule trajectories. Nano Lett 7:3138
Vanden Heuvel MGL, de Graaff MP, Lemay SG, Dekker C (2007) Electrophoresis of individual microtubules in microchannels. Proc Natl Acad Sci USA 104:7770–7775
Vanden Heuvel MGL, de Graaff MP, Dekker C (2008) Microtubule curvatures under perpendicular electric forces reveal a low persistence length. Proc Natl Acad Sci USA 105:7941
Venier P, Maggs AC, Carlier MF, Pantaloni D (1994) Analysis of microtubule rigidity using hydrodynamic flow and thermal fluctuations. J Biol Chem 269:13353
Wade RH, Chrétien D, Job D (1990) Characterization of microtubule protofilament numbers. How does the surface lattice accommodate?. J Mol Biol 212:775–786
Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA (2006) Insights into the mechanism of microtubule stabilization by taxol. Proc Natl Acad Sci USA 103:10166–10173
Acknowledgments
We acknowledge fruitful discussions with Francesco Pampaloni, Denis Chrétien, Thomas Surrey, Francois Nédélec, Jean-Francois Joanny, Sergey Obukhov, Linda Amos, and André E.X. Brown and thank Falko Ziebert for discussion and useful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
A. Polymorphic phase coherence length
In this section, we derive the formula \(l_{\phi}=\frac{N^{2}b}{8\pi^{2}}\left(2+{\rm e}^{2J/k_{\rm B}T}\right)\) for the polymorphic phase coherence length. To this end we want to calculate the distribution of double junctions that leads to angular orientation change \(\Updelta\Upphi\) on a scale l much larger than the tubulin dimer b, yet still much smaller than the total length, i.e., b ≪ l ≪ L. In this domain, at each cross-section we have three possibilities:
-
1.
State j = 0 with no double defect. The rotation angle \(\Updelta\Upphi\) is attached to the internal lattice rotation, \(\frac{\Updelta\Upphi}{b}-q_{0}=0.\)
-
2.
State j = −1 for a left-handed double defect; \(\Updelta\Upphi\) deviates from the internal twist: \(\frac{\Updelta\Upphi}{b}-q_{0}=-\frac{1}{b}\frac{2\pi}{N}.\)
-
3.
State j = +1 for a right-handed double defect with \(\frac{\Updelta\Upphi}{b}-q_{0}=+\frac{1}{b}\frac{2\pi}{N}.\)
On a length l we are throwing a three-sided dice l/b times and the total rotation of \(\Updelta\Upphi\) away from the optimal twist is \(\Updelta\Upphi-q_{0}l=\frac{2\pi}{N}\sum\nolimits_{n=1}^{l/b}j_{n}.\) The variation of the polymorphic phase with respect to the internal twist is then
For l/a ≫ 1 the law of large numbers implies that the random variable \(\Updelta\phi=\frac{1}{l}\frac{2\pi}{N}\sum\nolimits_{n=1}^{l/b}j_{n}\) becomes Gaussian distributed
with mean \(\left\langle \Updelta\phi\right\rangle =0\) and \(\left\langle \Updelta\phi^{2}\right\rangle =\left(\frac{1}{l}\frac{2\pi}{N}\right) ^{2}\left(\frac{l}{b}\right) \left\langle j^{2}\right\rangle \) (as \(\left\langle j_{n}j_{m}\right\rangle =\delta_{nm}\left\langle j^{2}\right\rangle \)). The average \(\left\langle j^{2}\right\rangle \) is given from the Boltzmann factors of the three different states \(p_{0}={\frac{1}{1+2{\rm e}^{-2\beta J}}}\) and \(p_{\pm1}=\frac{{\rm e}^{-2\beta J}}{1+2{\rm e}^{-2\beta J}},\) so that \(\left\langle j^{2}\right\rangle =\frac{2{\rm e}^{-2\beta J}}{1+2{\rm e}^{-2\beta J}}. \) We can now interpret the quantity \(1/\left(2\left\langle \Updelta \phi^{2}\right\rangle \right) \) as coming from an effective elastic energy over the interval l by writing \(\frac{\Updelta\phi^{2}}{2\left\langle \Updelta\phi^{2}\right\rangle }=\frac{1}{2}\beta C_{\phi}l\left(\Updelta \phi\right)^{2}\), which allows us to identify the effective stiffness
Note that this expression is valid for large enough J suppressing higher-order defects, i.e., in the limit when multiple double defects sitting on a single lattice site (i.e., | j| > 1) can be ignored.
B. The variation of the polymorphic modulus
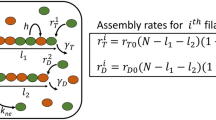
In this appendix we compute the energy variation due to a deviation of the polymorphic modulus |P| away from its optimal value | P *| minimizing the energy, i.e., the change of the number of switched PFs. We start with the energy density of a MT cross-section
whose minimum is reached for \(p^{\ast}=\frac{N}{2}-\frac{N}{2\pi }\arcsin\gamma. \) Assuming a continuous number of PFs, the energy of a state with \(p=p^{\ast}+\Updelta p\) switched PFs reads to quadratic order
where we used \(\cos(\pi-\arcsin\gamma)=-\sqrt{1-\gamma^{2}}.\) Therefore, the energy variation of a segment of length l reads
Now using \(\left\vert P\left(s\right) \right\vert =\left\vert \sin\left(\frac{\pi}{N}p\right) \right\vert /\sin(\pi/N)\) we can write the energy variation to the same (quadratic) order as
Therefore, any deviation of P from its optimum state P * is associated with an energy cost proportional to the length l of the region in the unfavorable state.
C. Persistence length(s)
A definition of the persistence length, often used in single-molecule experiments, is expressed in terms of the lateral deviation \(\overrightarrow {\rho}=(x(s),y\left(s\right))\) of a MT clamped at s = 0 from its attachment axis as \(l_{\rm p}^{\ast}\left(s\right) =(2/3)s^{3}/\left\langle \left(\overrightarrow{\rho}\left(s\right) -\left\langle \overrightarrow {\rho}\left(s\right) \right\rangle \right) ^{2}\right\rangle, \) where \(\left\langle \cdot \right\rangle \) is the statistical average. The equivalence of the x and y directions implies that \(l_{\rm p}^{\ast}\left(s\right) =1/3s^{3}/\left\langle \left(y\left(s\right) -\left\langle y\left(s\right) \right\rangle \right) ^{2}\right\rangle.\) The second often used alternative but more standard definition of the persistence length—the tangent persistence length—is related to the angular correlation \(l_{\rm p}\left(s-s^{\prime}\right) =\left\vert s-s^{\prime}\right\vert /V(s-s^{\prime})\) with variance \(V=\left\langle \left(\theta_{y}\left(s\right) -\theta_{y}\left(s^{\prime}\right) \right) ^{2}\right\rangle \) (by symmetry we have the same expression with θ x ). Whereas for an ideal WLC \(l_{\rm p}^{\ast}=l_{\rm p}=l_{\rm B}\) is position and definition independent, this is not the case for a polymorphic chain (see Fig. 13). For small angular deformations, the decoupling of the chain’s fluctuations into polymorphic and purely elastic contributions allows one to decompose the persistence length as l −1p = l −1pol + l −1B , this result being valid for both definitions of the persistence length.
We first focus on the first definition, for the clamped persistence length. In this case the polymorphic persistence length \(l_{\rm pol}^{\ast}(s)\) is given by
where y pol(s) is the lateral polymorphic displacement in the y direction. Integrating over the rotational zero mode readily implies \(\left\langle y_{\rm pol}\left(s\right) \right\rangle =0\) (see Eq. 18). From Eq. 19 one can write
with the angular correlation function \(G(s_{1},s_{2})=\left\langle \theta_{y,{\rm pol}}(s_{1})\theta_{y,{\rm pol}}(s_{2})\right\rangle \) given by the integration over the zero mode
of the angular correlation function at fixed value of ϕ 0, i.e., \(G_{0}(s_{1},s_{2},\phi_{0})=\left\langle \theta_{y,{\rm pol}}(s_{1})\theta _{y,{\rm pol}}(s_{2})\right\rangle |_{\phi_{0}}. \) This last expression, from the relation \(\theta_{y,{\rm pol}}(s)=\kappa_{0}\int_{0}^{s}\sin\left(\widetilde{\phi }\left(s^{\prime}\right) +q_{0}s^{\prime}+\phi_{0}\right) {\rm d}s^{\prime}\) (cf. Eq. 17), is explicitly given by
After integration over ϕ 0 and using the known result \(\left\langle \cos\left(\widetilde{\phi}\left(s_{1}\right) -\widetilde{\phi}\left(s_{2}\right) \right) \right\rangle ={\rm e}^{-\left\vert s_{1}-s_{2}\right\vert /2l_{\phi}}\) which results from the WLC-type probability distribution of the field \(\widetilde{\phi}, \) i.e., \(P[\widetilde{\phi}]\sim\exp(-\frac{l_{\phi}}{2} \int_{0}^{L}{\rm d}s\widetilde{\phi}^{\prime2})\), one obtains the rotational invariant correlation function in the form
Computation of the integrals in Eq. 38 gives finally the following expression for the polymorphic contribution to the transverse displacement:
with \({P}_{1}(s){ =24l}_{\phi}^{3}\left(1-6x+x^{2}\right) { -3l}_{\phi}\left(1+x-x^{2}-x^{3}\right) { s}^{2}\) \({ +}\left(1+3x+3x^{2}+x^{3}\right) { s}^{3}, { P}_{2}(s){ =24l}_{\phi}^{3}\left(1-6x+x^{2}\right) \) \({ +12l}_{\phi}^{2}\left(1-2x-3x^{2}\right) { s}\), and P 3(s) = 192l 4 ϕ q 0(1 − x) + 24l 3 ϕ q 0(3 + 2x − x 2)s, where we have introduced the notation x = 4l 2 ϕ q 20 .
From Eq. 39, we get the polymorphic persistence length \(l_{\rm pol}^{\ast}(s)\) defined in Eq. 35, and in turn the global persistence length \(l_{\rm p}^{\ast}(s)\) depicted in Fig. 13. Its physical interpretation is discussed in the main text.
We now consider the second definition of the persistence length \(l_{\rm p}\left(s-s^{\prime}\right) =\left\vert s-s^{\prime}\right\vert /V(s-s^{\prime}). \) From Eq. 38, the angular variance V pol can easily be evaluated as
The resulting persistence length l p (depicted in Fig. 13) shows a rich behavior similar to the persistence length \(l_{\rm p}^{\ast}\left(s\right) \) but displays a functional form distinct from the latter. However, as expected, both curves reach the same asymptotic value at very short and very long MT lengths.
D. Zero-mode dynamics
The evolution of the zero mode \(\phi_{0}\left(t\right) \) is given by Eq. 28 as
with a friction constant ξ tot = ξ int + ξ ext, where ξ ext is given by Eq. 27. The correlation function of the thermal white noise \(\Upgamma_{\phi}\left(s,t\right) \) is \(\left\langle \Upgamma_{\phi }\left(s,t\right) \Upgamma_{\phi}\left(s^{\prime},t^{\prime}\right) \right\rangle =D\delta(s-s^{\prime})\delta(t-t^{\prime})\) with a coefficient D that can be determined in the following manner. Notice first that ϕ 0 performs free Brownian motion and that its quadratic fluctuations necessarily satisfy the relation \(\left\langle \left(\phi_{0}\left(t\right) -\phi_{0}\left(0\right) \right) ^{2}\right\rangle ={ \frac{2k_{\rm B}T}{L\xi_{\rm tot}}}t. \) On the other hand, integrating Eq. 41 yields
and exploiting the white noise type auto-correlation of \(\Gamma\) one obtains \(\left\langle \left(\phi_{0}\left(t\right) -\phi_{0}\left(0\right) \right) ^{2}\right\rangle =\frac{D}{\xi_{\rm tot}^{2}L}t,\) from which we readily deduce D = 2ξ tot k B T, as expected from the fluctuation–dissipation theorem.
The relaxation time is generally given from the time correlation function <y pol(s, 0)y pol(s, t)> with the lateral position \(y_{\rm pol}(s,t)=\frac{\kappa_{0}}{q_{0}^{2}}(sq_{0}\cos\left(\phi_{0}\left(t\right) +\alpha\right) +\sin\left(\phi_{0}\left(t\right) +\alpha\right) -\sin\left(q_{0}s+\phi_{0}\left(t\right) +\alpha\right))\) obtained from Eq. 14 with l ϕ ≫ s. The average must first take into account all statistically equivalent values of angular orientations \(\alpha\in\left[ 0,2\pi\right] ,\) such that \(\left\langle y_{\rm pol}(s,0)y_{\rm pol}(s,t)\right\rangle =\int\nolimits_{0}^{2\pi}\left\langle y_{\rm pol}(s,0)y_{\rm pol}(s,t)\right\rangle _{\alpha}\frac{{\rm d}\alpha}{2\pi},\) and we obtain
with \(<y_{\rm pol}^{2}(s)>=\frac{\kappa_{0}^{2}}{q_{0}^{2}}\left(\frac{s^{2}}{2}+\frac{1-\cos(q_{0}s)}{q_{0}^{2}}-\frac{s\sin(q_{0}s)}{q_{0}} \right), \) corresponding to the static result (Eq. 39) in the limit s/l ϕ ≪ 1. With 42 defining a simple Gaussian random walk process, one straightforwardly obtains
with the relaxation time given by
E. Comment on MT surface attachment and the robustness of “wobbling”
Throughout this work we have assumed that the free rearrangement of the polymorphic lattice states is not significantly hindered by the covalent surface attachment of the MT, as e.g. performed by Pampaloni et al. (2006) and Taute et al. (2008). This assumption is integral to the “wobbling” motion and in turn to understanding the static and dynamic data scaling. It therefore deserves closer consideration.
In the experiments by Pampaloni et al. (2006) and Taute et al. (2008), the adsorbed MT part is attached to a gold (electron microscopy grid) surface via thiol groups. It is likely that \(\approx\)1–2 protofilaments will establish localized chemical contacts with the gold microgrid. While substantial perturbation of the dimer such as denaturation appears unlikely, it is unclear to what extent this procedure will perturb the inner (polymorphic) dynamics of the entire tubulin dimer units. In principle, one can anticipate two plausible scenarios that would interfere to a varying degree with the free “wobbling” motion:
-
S1.
Due to high cooperativity (large coupling J) the polymorphic state transition can propagate within a certain penetration depth into the adsorbed (straight-planar) MT section.
-
S2.
The cooperativity is too weak to compete with the constraints imposed by the surface (including chemical perturbations), and the polymorphic transition does not propagate into the straight adsorbed MT section.
In both cases we have a nonvanishing deflection angle between a forced (adsorbed) planar section and the free helical section direction, effectively causing the characteristic MT “kink” at the surface interface. However, the rotational mobility of this “kink” (wobbling mode) which is integral to our theory will be affected in a slightly different manner.
If in case S1, in the adsorbed section, the polymorphic order parameter P can rearrange to some extent (by switching the monomer states without causing detectable deformation) except for possibly in the few surface-interacting dimers, then the effects of the “wobbling” motion will be hindered only mildly in the following sense. To retrieve the anomalous lateral fluctuations it is indeed enough for the wobbling angle ϕ 0 to move freely in a certain nonvanishing angular interval. A single complete or multiple rotations of the order parameter \(\vec{P}\) are not strictly necessary for the “hinge” effect, and they are in fact equivalent in lateral projection (as in experiment) to the motion of the wobbling angle ϕ 0 in the smaller interval [−π/2, +π/2]. Note that even intervals smaller than this will lead to a similar phenomenology (in particular, dynamic and static variable scalings with length). Thus, the conical hinge-like motion is in a sense robust with respect to a limited local rotational hindrance perturbation in the adsorbed region.
In scenario S2 the situation is somehow simpler as the polymorphic dynamics of the adsorbed region is not involved in the process (the polymorphic order parameter vanishes there: \(\vec{P}=0\)). Wobbling is realized through a coherent rearrangement of the free MT section alone, without strong coupling to the adsorbed region.
Although both attachment scenarios S1 and S2 appear to some extent plausible, at present it is difficult to make reliable statements about their respective likelihood. In fact only a posteriori can we cautiously state that, based on the experimental static and dynamic measurement evidence, the chain “wobbles” to a high enough extent to display the effects that we observe.
Rights and permissions
About this article
Cite this article
Mohrbach, H., Johner, A. & Kulić, I.M. Cooperative lattice dynamics and anomalous fluctuations of microtubules. Eur Biophys J 41, 217–239 (2012). https://doi.org/10.1007/s00249-011-0778-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0778-0