Abstract
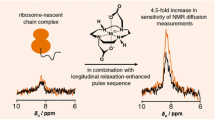
Pulsed-field gradient nuclear magnetic resonance has seen an increase in applications spanning a broad range of disciplines where molecular translational diffusion properties are of interest. The current study introduces and experimentally evaluates the measurement of translational diffusion coefficients of 15N-enriched biomolecules using a 1H-15N HMQC-filtered band-selective excitation short transient (BEST) sequence as an alternative to the previously described SOFAST-XSTE sequence. The results demonstrate that accurate translational diffusion coefficients of 15N-labelled peptides and proteins can be obtained using this alternative 1H-15N HMQC-filtered BEST sequence which is implementable on NMR spectrometers equipped with probes fitted with a single-axis field gradient, including most cryoprobes dedicated to bio-NMR. The sequence is of potential use for direct quantification of protein or peptide translational diffusion within complex systems, such as in mixtures of macromolecules, crowded solutions, membrane-mimicking media and in bicontinuous cubic phases, where conventional sequences may not be readily applicable due to the presence of intense signals arising from sources other than the protein or peptide under investigation.







Similar content being viewed by others
References
Ali FE, Separovic F, Barrow CJ, Yao S, Barnham KJ (2006) Copper and zinc mediated oligomerisation of A beta peptides. Int J Pept Res Ther 12:153–164
Altieri AS, Hinton DP, Byrd RA (1995) Association of biomolecular systems via pulsed-field gradient NMR self-diffusion measurements. J Am Chem Soc 117:7566–7567
Andersson A, Almqvist J, Hagn F, Maler L (2004) Diffusion and dynamics of penetratin in different membrane mimicking media. BBA Biomembranes 1661:18–25
Andrec M, Prestegard JH (1997) Quantitation of chemical exchange rates using pulsed-field-gradient diffusion measurements. J Biomol NMR 9:136–150
Augustyniak R, Ferrage F, Paquin R, Lequin O, Bodenhausen G (2011) Methods to determine slow diffusion coefficients of biomolecules. Applications to engrailed 2, a partially disordered protein. J Biomol NMR 50:209–218
Barchi JJ, Grasberger B, Gronenborn AM, Clore GM (1994) Investigation of the backbone dynamics of the Igg-binding domain of streptococcal protein-G by heteronuclear 2-dimensional H-1–N-15 nuclear-magnetic-resonance spectroscopy. Protein Sci 3:15–21
Barhoum S, Palit S, Yethiraj A (2016) Diffusion NMR studies of macromolecular complex formation, crowding and confinement in soft materials. Prog Nucl Mag Res Sp 94–95:1–10
Bocian W, Sitkowski J, Tarnowska A, Bednarek E, Kawecki R, Kozminski W, Kozerski L (2008) Direct insight into insulin aggregation by 2D NMR complemented by PFGSE NMR. Proteins 71:1057–1065
Brand T, Cabrita EJ, Morris GA, Gunther R, Hofmann HJ, Berger S (2007) Residue-specific NH exchange rates studied by NMR diffusion experiments. J Magn Reson 187:97–104
Buevich AV, Baum J (2002) Residue-specific real-time NMR diffusion experiments define the association states of proteins during folding. J Am Chem Soc 124:7156–7162
Callaghan PT, Legros MA, Pinder DN (1983) The measurement of diffusion using deuterium pulse field gradient nuclear magnetic resonance. J Chem Phys 79:6372–6381
Chou JJ, Baber JL, Bax A (2004) Characterization of phospholipid mixed micelles by translational diffusion. J Biomol NMR 29:299–308
Dehner A, Kessler H (2005) Diffusion NMR spectroscopy: folding and aggregation of domains in p53. ChemBioChem 6:1550–1565
Didenko T, Boelens R, Rudiger SGD (2011) 3D DOSY-TROSY to determine the translational diffusion coefficient of large protein complexes. Protein Eng Des Sel 24:99–103
Dingley AJ, Mackay JP, Chapman BE, Morris MB, Kuchel PW, Hambly BD, King GF (1995) Measuring protein self-association using pulsed-field-gradient NMR spectroscopy: application to myosin light chain 2. J Biomol NMR 6:321–328
Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Bio 6:197–208
Eriksson PO, Lindblom G (1993) Lipid and water diffusion in bicontinuous cubic phases measured by NMR. Biophys J 64:129–136
Gossert AD, Jahnke W (2016) NMR in drug discovery: a practical guide to identification and validation of ligands interacting with biological macromolecules. Prog Nucl Mag Res Sp 97:82–125
Horst R, Horwich AL, Wuthrich K (2011) Translational diffusion of macromolecular assemblies measured using transverse-relaxation-optimized pulsed field gradient NMR. J Am Chem Soc 133:16354–16357
Jansma AL, Kirkpatrick JP, Hsu AR, Handel TM, Nietlispach D (2010) NMR analysis of the structure, dynamics, and unique oligomerization properties of the chemokine CCL27. J Biol Chem 285:14424–14437
Larkin TJ, Garvey CJ, Shishmarev D, Kuchel PW, Momot KI (2017) Na+ and solute diffusion in aqueous channels of Myverol bicontinuous cubic phase: pGSE NMR and computer modelling. Magn Reson Chem 55:464–471
Lescop E, Schanda P, Brutscher B (2007) A set of BEST triple-resonance experiments for time-optimized protein resonance assignment. J Magn Reson 187:163–169
Li CG, Wang YQ, Pielak GJ (2009) Translational and rotational diffusion of a small globular protein under crowded conditions. J Phys Chem B 113:13390–13392
McLachlan GD, Cahill SM, Girvin ME, Almo SC (2007) Acid-induced equilibrium folding intermediate of human platelet profilin. Biochemistry 46:6931–6943
Meikle TG, Yao S, Zabara A, Conn CE, Drummond CJ, Separovic F (2017) Predicting the release profile of small molecules from within the ordered nanostructured lipidic bicontinuous cubic phase using translational diffusion coefficients determined by PFG-NMR. Nanoscale 9:2471–2478
Melnikova DL, Skirda VD, Nesmelova IV (2017) Effect of intrinsic disorder and self-association on the translational diffusion of proteins: the case of alpha-casein. J Phys Chem B 121:2980–2988
Nesmelova IV, Idiyatullin D, Mayo KH (2004) Measuring protein self-diffusion in protein-protein mixtures using a pulsed gradient spin-echo technique with WATERGATE and isotope filtering. J Magn Reson 166:129–133
Price WS (2009) NMR studies of translational motion: principles and applications. Cambridge University Press, Cambridge, New York
Rajagopalan S, Chow C, Raghunathan V, Fry CG, Cavagnero S (2004) NMR spectroscopic filtration of polypeptides and proteins in complex mixtures. J Biomol NMR 29:505–516
Roosen-Runge F, Hennig M, Zhang FJ, Jacobs RMJ, Sztucki M, Schober H, Seydel T, Schreiber F (2011) Protein self-diffusion in crowded solutions. P Natl Acad Sci USA 108:11815–11820
Rothe M, Gruber T, Groger S, Balbach J, Saalwachter K, Roos M (2016) Transient binding accounts for apparent violation of the generalized Stokes-Einstein relation in crowded protein solutions. Phys Chem Chem Phys 18:18006–18014
Schanda P (2009) Fast-pulsing longitudinal relaxation optimized techniques: enriching the toolbox of fast biomolecular NMR spectroscopy. Prog Nucl Mag Res Sp 55:238–265
Schanda P, Brutscher B (2005) Very fast two-dimensional NMR spectroscopy for real-time investigation of dynamic events in proteins on the time scale of seconds. J Am Chem Soc 127:8014–8015
Sethi A, Bruell S, Patil N, Hossain MA, Scott DJ, Petrie EJ, Bathgate RAD, Gooley PR (2016) The complex binding mode of the peptide hormone H2 relaxin to its receptor RXFP1. Nat Commun 7:11344
Shukla M, Dorai K (2011) Resolving overlaps in diffusion encoded spectra using band-selective pulses in a 3D BEST-DOSY experiment. J Magn Reson 213:69–75
Sillerud LO, Larson RS (2012) Advances in nuclear magnetic resonance for drug discovery. Methods Mol Biol 910:195–266
Stejskal EO, Tanner JE (1965) Spin Diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys 42:288–292
Susac L, Horst R, Wuthrich K (2014) Solution-NMR characterization of outer-membrane protein A from E. coli in lipid bilayer nanodiscs and detergent micelles. ChemBioChem 15:995–1000
Tanner JE (1970) Use of stimulated echo in NMR-diffusion studies. J Chem Phys 52:2523–2526
Wahlstrom A, Cukalevski R, Danielsson J, Jarvet J, Onagi H, Rebek J, Linse S, Graslund A (2012) Specific binding of a beta-cyclodextrin dimer to the amyloid beta peptide modulates the peptide aggregation process. Biochemistry 51:4280–4289
Wang YQ, Li CG, Pielak GJ (2010) Effects of proteins on protein diffusion. J Am Chem Soc 132:9392–9397
Wang YQ, Benton LA, Singh V, Pielak GJ (2012) Disordered protein diffusion under crowded conditions. J Phys Chem Lett 3:2703–2706
Wu KP, Kim S, Fela DA, Baum J (2008) Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: implication for aggregation. J Mol Biol 378:1104–1115
Yan JL, Kline AD, Mo HP, Zartler ER, Shapiro MJ (2002) Epitope mapping of ligand-receptor interactions by diffusion NMR. J Am Chem Soc 124:9984–9985
Yao S, Howlett GJ, Norton RS (2000) Peptide self-association in aqueous trifluoroethanol monitored by pulsed field gradient NMR diffusion measurements. J Biomol NMR 16:109–119
Yao S, Cherny RA, Bush AI, Masters CL, Barnham KJ (2004) Characterizing bathocuproine self-association and subsequent binding to Alzheimer’s disease amyloid beta-peptide by NMR. J Pept Sci 10:210–217
Yao S, Babon JJ, Norton RS (2008) Protein effective rotational correlation times from translational self-diffusion coefficients measured by PFG-NMR. Biophys Chem 136:145–151
Yao S, Weber DK, Separovic F, Keizer DW (2014) Measuring translational diffusion coefficients of peptides and proteins by PFG-NMR using band-selective RF pulses. Eur Biophys J 43:331–339
Yao S, Lee EF, Pettikiriarachchi A, Evangelista M, Keizer DW, Fairlie WD (2016) Characterisation of the conformational preference and dynamics of the intrinsically disordered N-terminal region of Beclin 1 by NMR spectroscopy. BBA Proteins Proteom 1864:1128–1137
Zabara A, Meikle TG, Trenker R, Yao S, Newman J, Peat TS, Separovic F, Conn CE, Call MJ, Call ME, Landau EM, Drummond CJ (2017) Lipidic cubic phase-induced membrane protein crystallization: interplay between lipid molecular structure, mesophase structure and properties, and crystallogenesis. Cryst Growth Des 17:5667–5674
Zhang XC, Perugini MA, Yao S, Adda CG, Murphy VJ, Low A, Anders RF, Norton RS (2008) Solution conformation, backbone dynamics and lipid interactions of the intrinsically unstructured malaria surface protein MSP2. J Mol Biol 379:105–121
Acknowledgements
We thank Dr Aitor Moreno of Bruker for the source code of the 1H-15N HSQC-edited PFG-NMR sequence (Fig. S3, Supplemental Materials).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, S., Meikle, T.G., Sethi, A. et al. Measuring translational diffusion of 15N-enriched biomolecules in complex solutions with a simplified 1H-15N HMQC-filtered BEST sequence. Eur Biophys J 47, 891–902 (2018). https://doi.org/10.1007/s00249-018-1311-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-018-1311-5