Abstract
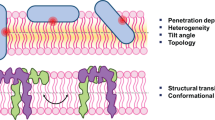
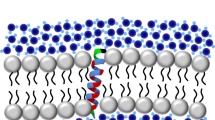
The fluorescence from tryptophan contains valuable information about the environment local to the indole side-chain. This environment sensitivity coupled with the ability to synthetically or genetically incorporate a single tryptophan residue at specific sites in a polypeptide sequence has provided the membrane biophysicist with powerful tools for examining the structure and dynamics of membrane peptides and proteins. Here we briefly review the use of site-specific tryptophan fluorescence spectroscopy to probe aspects of peptide orientation, structure, and dynamics in lipid bilayers, focusing on recent developments in the literature.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Revised version: 7 August 2001
Electronic Publication
Rights and permissions
About this article
Cite this article
Clayton, A.H., Sawyer, W.H. Site-specific tryptophan fluorescence spectroscopy as a probe of membrane peptide structure and dynamics. Eur Biophys J 31, 9–13 (2002). https://doi.org/10.1007/s002490100182
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s002490100182