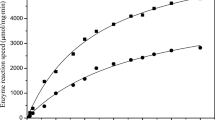
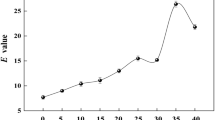
Abstract
In the present study, we used gene manipulation to construct a recombinant Aspergillus oryzae strain overexpressing lipase and investigated its application to the optical resolution of chiral compounds. A. oryzae niaD300, which was derived from the wild-type strain RIB40, was used as the host strain. The tglA gene, which encodes a triacylglycerol lipase, was cloned from the A. oryzae niaD300 chromosomal genome, then reintroduced, with and without a secretion-signal sequence, into the genome and expressed under the control of the improved glaA promoter of plasmid pNGA142. The resulting recombinant strain overexpressing A. oryzae lipase was immobilized within biomass-support particles and used as a whole-cell biocatalyst. The immobilized lipase-overexpressing strain with secretion-signal sequence showed high activity and was used to selectively synthesize (R)-1-phenylethyl acetate from (RS)-1-phenylethanol and vinyl acetate. After 48 h reaction at 30°C with molecular sieve 4A, the yield and enantiomeric excess (%ee) of (R)-1-phenylethyl acetate reached approximately 90 and 95%ee, respectively. The whole-cell biocatalyst for optical resolution of chiral compounds produced in this study maintained its activity over 25 batch-reaction cycles.






Similar content being viewed by others
References
Balcao VM, Paiva AL, Malcata X (1996) Bioreactors with immobilized lipases: state of the art. Enzyme Microb Technol 18:392–416
Ban K, Kaieda M, Matsumoto T, Kondo A, Fukuda H (2001) Whole cell biocatalyst for biodiesel fuel production utilizing Rhizopus oryzae cells immobilized within biomass support particles. Biochem Eng J 8:39–43
Ban K, Hama S, Nishizuka K, Kaieda M, Matsumoto T, Kondo A, Noda H, Fukuda H (2002) Repeated use of whole-cell biocatalysts immobilized within biomass support particles for biodiesel fuel production. J Mol Catal B 17:157–165
Fernandez-Lorente G, Palomo JM, Fuentes M, Mateo C, Guisan JM, Fernandez-Lafuente R (2003) Self-assembly of Pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol Bioeng 82:232–237
Gomi K, Iimura Y, Hara S (1987) Integrative transformation of Aspergillus oryzae with a plasmid containing the Aspergillus nidulans argB gene. Agric Biol Chem 51:2549–2555
Han SJ, Back JH, Yoon MY, Shin PK, Cheong CS, Sung MH, Hong SP, Chung IY, Han YS (2003) Expression and characterization of a novel enantioselective lipase from Acinetobacter species SY-01. Biochimie 85:501–510
Huge-Jensen B, Andreasen F, Christensen T, Christensen M, Thim L, Boel E (1989) Rhizomucor miehei triglyceride lipase is processed and secreted from transformed Aspergillus oryzae. Lipids 24:781–785
Kim MJ, Ahn Y, Park J (2002) Dynamic kinetic resolutions and asymmetric transformations by enzymes coupled with metal catalysis. Curr Opin Biotechnol 13:578–587
Kitamoto K (2002) Molecular biology of the koji mold. Adv Appl Microbiol 51:129–153
Lee SG, Lee JO, Yi JK, Kim BG (2002) Production of cytidine 5′-monophosphate N-acetylneuraminic acid using recombinant Escherichia coli as a biocatalyst. Biotechnol Bioeng 80:516–524
Liu Y, Hama H, Fujita Y, Kondo A, Inoue Y, Kimura A, Fukuda H (1999) Production of S-lactoylglutathione by high activity whole cell biocatalysts prepared by permeabilization of recombinant Saccharomyces cerevisiae with alcohols. Biotechnol Bioeng 64:54–60
Martinez MB, Flickinger MC, Nelsestuen GL (1999) Steady-state enzyme kinetics in the Escherichia coli periplasm: a model of a whole cell biocatalyst. J Biotechnol 71:59–66
Matsumoto T, Takahashi S, Kaieda M, Ueda M, Tanaka A, Fukuda H, Kondo A (2001) Yeast whole-cell biocatalyst constructed by intracellular overproduction of Rhizopus oryzae lipase is applicable to biodiesel fuel production. Appl Microbiol Biotechnol 57:515–520
Minetoki T, Nunokawa Y, Gomi K, Kitamoto K, Kumagai C, Tamura G (1996) Deletion analysis of promoter elements of the Aspergillus oryzae agdA gene encoding α-glucosidase. Curr Genet 30:432–438
Minetoki T, Ozeki K, Kumagai C, Gomi K, Iimura Y (1997) An expression system based on the improved promoter containing multiple copies of the conserved sequence in the amylase genes of Aspergillus oryzae. Proceedings of the 19th Fungal Genetics Conference, California, p 68
Minetoki T, Kumagai C, Gomi K, Kitamoto K, Takahashi K (1998) Improvement of promoter activity by the introduction of multiple copies of the conserved region III sequence, involved in the efficient expression of Aspergillus oryzae amylase-encoding genes. Appl Microbiol Biotechnol 50:459–467
Nakashima T, Fukuda H, Kyotani S (1988) Culture conditions for intracellular lipase production by Rhizopus chinensis and its immobilization within biomass support particles. J Ferment Technol 66:441–448
Tsuchiya K, Tada S, Gomi K, Kitamoto K, Kumagai C, Tamura G (1992) Deletion analysis of the Taka-amylase a gene promoter using a homologous transformation system in Aspergillus oryzae. Biosci Biotechnol Biochem 56:1849–1853
Wang DL, Nag A, Lee GC, Shaw JF (2002) Factors affecting the resolution of dl-menthol by immobilized lipase-catalyzed esterification in organic solvent. J Agric Food Chem 50:262–265
Yasokawa D, Shimizu T, Nakagawa R, Ikeda T, Nagashima K (2003) Cloning, sequencing, and heterologous expression of a cellobiohydrolase cDNA from the basidiomycete Corticium rolfsii. Biosci Biotechnol Biochem 67:1319–1326
Acknowledgements
We are grateful to Toshitaka Minetoki (Ozeki Co.) for providing Aspergillus oryzae strain niaD300, plasmid pNGA142, and useful advice. This work was supported in part by grants-in-aid for JSPS Fellows from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaieda, M., Nagayoshi, M., Hama, S. et al. Enantioselective transesterification using immobilized Aspergillus oryzae overexpressing lipase. Appl Microbiol Biotechnol 65, 301–305 (2004). https://doi.org/10.1007/s00253-004-1590-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1590-x