Abstract
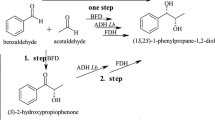
Enantioselective biotransformation of dl-1,2-propanediol to d-2-hydroxypropanic acid was first reported by the authors. In the biooxidation process, there were some by-product formed and thus influenced the e.e. value and output of the acid. Restricting oxygen in the reaction system and offering additional proton receptor to the system displayed approving effect. The latter method constructed regeneration cycle system of coenzyme. In the article, the bioreduction of pinacolone was coupled to the enantioselective oxidation. Yield of the acid was increased by 36% and e.e. value of the product approached 99%.








Similar content being viewed by others
References
Adachi K, Sonboon T, Toyama H, Matsushita K (2003) Quinate dehydrogenase-producing acetic acid bacteria for manufacture of shikimic acid, Jpn Kokai Tokkyo Koho JP 2003070493 A2 11
De Cabo R, Cabello R, Rios M, Lopez-Lluch G, Ingram DK, Lane MA, Navas P (2004) Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol 39:297–304
Ferrieri RA (2002) Application of chiral critical clusters to asymmetric synthesis, US 6486355 B1
Iwamoto M, Kawada H, Tanaka T, Nakada M (2003) Preparation of new chiral building blocks via asymmetric catalysis. Tetrahedron Lett 44:7239–7243
Kawaguchi M, Hibino T, Takenaka S, Hattori T, Arima M (2005) Colored biodegradable plastics with good safety and discoloration resistance, Jpn Kokai Tokkyo Koho JP 2005068209 A2
Laumen K, Kittelman M, Ghisalba O (2002) Chemo-enzymatic approaches for the creation of novel chiral building blocks and reagents for pharmaceutical applications, J Mol Catal B Enzym 19:55–66
Liu SQ (2002) Malolactic fermentation in wine — beyond deacidification. J Appl Microbiol 92:589–601
Molinari F, Gandolfi R, Leon R, Prazeres DMF (1999) Biotransformations in two-liquid phase systems: production of phenylacetaldehyde by acetic acid bacteria. Enzyme Microb Technol 25:729–735
Pawelkiewicz J, Zagalak B (1965) Enzymic conversion of glycerol into β-hydroxy-propionaldehyde in a cell-free extract from Aerobacter aerogenes. Acta Biochim Pol 12:207–218
Peeters J, Palmans ARA, Veld M, Scheijen F, Heise A, Meijer EW (2004) Cascade synthesis of chiral block copolymers combining lipase catalyzed ring opening polymerization and atom transfer radical polymerization. Biomacromolecules 5:1862–1868
Renac R (2001) Biotechnology applied to chemistry. Actual Chim 9:3–8
Ruether T, Hultgren VM, Timko BP, Bond AM, Jackson WR, Wedd AG (2003) Electrochemical investigation of photooxidation processes promoted by sulfo-polyoxometalates: coupling of photochemical and electrochemical processes into an effective catalytic cycle. J Am Chem Soc 125:10133–10143
Sanghani PC, Robinson H, Bosron WF, Hurley TD (2002) Human Glutathione-Dependent Formaldehyde Dehydrogenase, Structures of Apo, Binary, and Inhibitory Ternary Complexes. Biochemistry 41:10778–10786
Schulz T, Schmid RD, Pleiss J (2001) Structural basis of stereoselectivity in Candida rugosa lipase-catalyzed hydrolysis of secondary alcohols. J Mol Model 7:265–270
Shimizu S (2003) Biochemical and applied studies of vitamin production by microorganisms. Bitamin 77:131–145
Song YC, Okamoto S, Sato F (2003) An efficient synthesis of optically active (2R,3R)-2-methyl-3-[(1R)-1-methylprop-2-enyl] cyclopentanone, a useful chiral building block for synthesis of vitamin D and steroids. Tetrahedron Lett 44:2113–2115
Su W, Chang ZY, Gao KL, Wei DZ (2004) Enantioselective oxidation of racemic 1,2-propanediol to D-(-)-lactic acid by Gluconobacter oxydans. Tetrahedron: Asymmetry 15:1275–1277
Tachihara T, Kitahara T (2003) Total synthesis of (+)-epiepoformin, (+)-epiepoxydon and (+)-bromoxone employing a useful chiral building block, ethyl (1R,2S)-5,5-ethylenedioxy-2-hydroxycyclohexanecarboxylate. Tetrahedron 59:1773–1780
Tanaka N (2004) Biodegradable plastics-recent progress. Kino Zairyo 24:45–48 (Japanese)
Yasutake Y, Watanabe S, Yao M, Takada Y, Fukunaga N, Tanaka I (2003) Crystal structure of the monomeric isocitrate dehydrogenase in the presence of NADP+: Insight into the cofactor recognition, catalysis, and evolution. J Biol Chem 278:36897–36904
Acknowledgement
The financial support for scientific research from Key Disciplinary Foundation of Shanghai is fully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, K., Song, Q. & Wei, D. Coupling of enantioselective biooxidation of dl-1,2-propanediol and bioreduction of pinacolone via regeneration cycle of coenzyme. Appl Microbiol Biotechnol 71, 819–823 (2006). https://doi.org/10.1007/s00253-005-0231-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0231-3