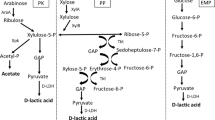
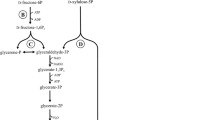
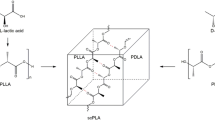
Abstract
Lactic acid (LA) is an important and versatile chemical that can be produced from renewable resources such as biomass. LA is used in the food, pharmaceutical, and polymers industries and is produced by microorganism fermentation; however, most microorganisms cannot directly utilize biomass such as starchy materials and cellulose. Here, we summarize LA production using several kinds of genetically modified microorganisms, such as LA bacteria, Escherichia coli, Corynebacterium glutamicum, and yeast. Using gene manipulation and metabolic engineering, the yield and optical purity of LA produced from biomass has been significantly improved. In this review, the drawbacks as well as improvements of LA production by fermentation is discussed.



Similar content being viewed by others
References
Adachi E, Torigoe M, Sugiyama M, Nikawa J, Shimizu K (1998) Modification of metabolic pathways of Saccharomyces cerevisiae by the expression of lactate dehydrogenase and deletion of pyruvate decarboxylase genes for the lactic acid fermentation at low pH value. J Ferment Bioeng 86:284–289
Adsul M, Khire J, Bastawde K, Gokhale D (2007) Production of lactic acid from cellobiose and cellotriose by Lactobacillus delbrueckii mutant Uc-3. Appl Environ Microbiol 73:5055–5057
Bianchi MM, Brambilla L, Protani F, Liu CL, Lievense J, Porro D (2001) Efficient homolactic fermentation by Kluyveromyces lactis strains defective in pyruvate utilization and transformed with the heterologous LDH gene. Appl Envion Microbiol 67:5621–5625
Bustos G, Moldes AB, Cruz JM, Domínguez JM (2005) Influence of the metabolism pathway on lactic acid production from hemicellulosic trimming vine shoots hydrolyzates using Lactobacillus pentosus. Biotechnol Prog 21:793–798
Chaillou S, Bor YC, Batt CA, Postma PW, Pouwels PH (1998) Molecular cloning and functional expression in Lactobacillus plantarum 80 of xylT, encoding the D-xylose-H+ symporter of Lactobacillus brevis. Appl Environ Microbiol 64:4720–4728
Chang DE, Jung HC, Rhee JS, Pan JG (1999) Homofermentative production of D- or L-lactate in metabolically engineered Escherichia coli RR1. Appl Environ Microbiol 65:1384–1389
Christensen CH, Rass-Hansen J, Marsden CC, Taarning E, Egeblad K (2008) The renewable chemicals industry. ChemSusChem 1:283–289
Dequin S, Barre P (1994) Mixed lactic acid-alcoholic fermentation by Saccharomyces cerevisiae expressing the Lactobacillus casei L(+)-LDH. Biotechnology 12:173–177
Dequin S, Baptista E, Barre P (1999) Acidification of grape musts by Saccharomyces cerevisiae wine yeast strains genetically engineered to produce lactic acid. Am J Enol Vitic 50:45–50
Dodds DR, Gross RA (2007) Chemicals from biomass. Science 318:1250–1251
Gao MT, Shimamura T, Ishida N, Takahashi H (2009) Application of metabolically engineered Saccharomyces cerevisiae to extractive lactic acid fermentation. Biochem Eng J 44:251–255
Giraud E, Champailler A, Raimbault M (1994) Degradation of raw starch by a wild amylolytic strain of Lactobacillus plantarum. Appl Environ Microbiol 60:4319–4323
Guyot JP, Calderon M, Morlon-Guyot J (2000) Effect of pH control on lactic acid fermentation of starch by Lactobacillus manihotivorans LMG 18010T. J Appl Microbiol 88:176–182
Helanto M, Kiviharju K, Leisola M, Nyyssölä A (2007) Metabolic engineering of Lactobacillus plantarum for production of L-ribulose. Appl Environ Microbiol 73:7083–7091
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104:155–172
Hofvendahl K, Hahn-Hägerdal B (1997) L-lactic acid production from whole wheat flour hydrolysate using strains of Lactobacilli and Lactococci. Enzyme Microb Technol 20:301–307
Hofvendahl K, Hahn-Hägerdal B (2000) Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb Technol 26:87–107
Hohmann S (1991) Characterization of PDC6, a third structural gene for pyruvate decarboxylase in Saccharomyces cerevisiae. J Bacteriol 173:7963–7969
Hohmann S, Cederberg H (1990) Autoregulation may control the expression of yeast pyruvate decarboxylase structural genes PDC1 and PDC5. Eur J Biochem 188:615–621
Ikada Y, Jamshidi K, Tsuji H, Hyon SH (1987) Stereocomplex formation between enantiomeric poly (lactides). Macromolecules 20:904–906
Ishida N, Saitoh S, Tokuhiro K, Nagamori E, Matsuyama T, Kitamoto K, Takahashi H (2005) Efficient production of L-lactic acid by metabolically engineered Sacchromyces cerevisiae with a genome-integrated L-lactate dehydrogenase gene. Appl Environ Microbiol 71:1964–1970
Ishida N, Saitoh S, Onishi T, Tokuhiro K, Nagamori E, Kitamoto K, Takahashi H (2006a) The effect of pyruvate decarboxylase gene knockout in Sacchromyces cerevisiae on L-lactic acid production. Biosci Biotechnol Biochem 70:1148–1153
Ishida N, Suzuki T, Tokuhiro K, Nagamori E, Onishi T, Saitoh S, Kitamoto K, Takahashi H (2006b) D-lactic acid production by metabolically engineered Sacchromyces cerevisiae. J Biosci Bioeng 101:172–177
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Krämer R, Linke B, McHardy AC, Meyer F, Mockel B, Pfefferle W, Pühler A, Rey DA, Ruckert C, Rupp O, Sahm H, Wendisch VF, Wiegrabe I, Tauch A (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol 104:5–25
Katahira S, Mizuike A, Fukuda H, Kondo A (2006) Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide-assimilating yeast strain. Appl Microbiol Biotechnol 72:1136–1143
Kawaguchi T, Enoki T, Tsurumaki S, Sumitani J, Ueda M, Ooi T, Arai M (1996) Cloning and sequencing of the cDNA encoding β-glucosidase 1 from Aspergillus aculeatus. Gene 173:287–288
Kawaguchi H, Vertès AA, Okino S, Inui M, Yukawa H (2006) Engineering of a xylose metabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72:3418–3428
Kawaguchi H, Sasaki M, Vertès AA, Inui M, Yukawa H (2008) Engineering of an L-arabinose metabolic pathway in Corynebacterium glutamicum. Appl Microbiol Biotechnol 77:1053–1062
Kotrba P, Inui M, Yukawa H (2001) The ptsI gene encoding enzyme I of the phosphotransferase system of Corynebacterium glutamicum. Biochem Biophys Res Commun 289:1307–1313
Kotrba P, Inui M, Yukawa H (2003) A single V317A or V317M substitution in Enzyme II of a newly identified β-glucoside phosphotransferase and utilization system of Corynebacterium glutamicum R extends its specificity towards cellobiose. Microbiology 149:1569–1580
Leskovac V, Trivić S, Pericin D (2002) The three zinc-containing alcohol dehydrogenases from baker’s yeast, Saccharomyces cerevisiae. FEMS Yeast Res 2:481–494
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8
Litchfield JH (1996) Microbiological production of lactic acid. Adv Appl Microbiol 42:45–95
Matsui Y, Okada S, Uchimura T, Kondo A, Satoh E (2007) Determination and analysis of the starch binding domain of Streptococcus bovis 148 raw-starch-hydrolyzing α-amylase. J Appl Glycosci 54:217–222
Narita J, Nakahara S, Fukuda H, Kondo A (2004) Efficient production of L-(+)-lactic acid from raw starch by Streptococcus bovis 148. J Biosci Bioeng 97:423–425
Ohara H, Owaki M, Sonomoto K (2006) Xylooligosaccharide fermentation with Leuconostoc lactis. J Biosci Bioeng 101:415–420
Okano K, Kimura S, Narita J, Fukuda H, Kondo A (2007) Improvement in lactic acid production from starch using α-amylase-secreting Lactococcus lactis cells adapted to maltose or starch. Appl Microbiol Biotechnol 75:1007–1013
Okano K, Yoshida S, Tanaka T, Fukuda H, Kondo A (2009a) Homo D-lactic acid fermentation from arabinose by redirection of phosphoketolase pathway to pentose phosphate pathway in L-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microbiol 75(15):5175–5178
Okano K, Zhang Q, Shinkawa S, Yoshida S, Tanaka T, Fukuda H, Kondo A (2009b) Efficient production of optically pure D-lactic acid from raw corn starch by using genetically modified L-lactate dehydrogenase gene-deficient and α-amylase-secreting Lactobacillus plantarum strain. Appl Environ Microbiol 75:462–467
Okano K, Zhang Q, Yoshida S, Tanaka T, Ogino C, Fukuda H, Kondo A (2009c) D-Lactic acid production from cellooligosaccharides and β-glucan using L-LDH gene-deficient and endoglucanase-secreting Lactobacillus plantarum. Appl Microbiol Biotechnol (in press)
Okino S, Inui M, Yukawa H (2005) Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 68:475–480
Okino S, Suda M, Fujikura K, Inui M, Yukawa H (2008) Production of D-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 78:449–454
Porro D, Brambilla L, Ranzi BM, Martegani E, Alberghina L (1995) Development of metabolically engineered Saccharomyces cerevisiae cells for the production of lactic acid. Biotechnol Prog 11:294–298
Saitoh S, Mieno Y, Nagashima T, Kumagai C, Kitamoto K (1996) Breeding of a new type of baker’s yeast by δ-integration for overproduction of glucoamylase using a homothallic yeast. J Ferment Bioeng 81:98–103
Saitoh S, Ishida N, Onishi T, Tokuhiro K, Nagamori E, Kitamoto K, Takahashi H (2005) Genetically engineered wine yeast produces a high concentration of L-lactic acid of extremely high optical purity. Appl Environ Microbiol 71:2789–2792
Sasaki M, Jojima T, Inui M, Yukawa H (2008) Simultaneous utilization of D-cellobiose, D-glucose, and D-xylose by recombinant Corynebacterium glutamicum under oxygen-deprived conditions. Appl Microbiol Biotechnol 81:691–699
Satoh E, Niimura Y, Uchimura T, Kozaki M, Komagata K (1993) Molecular cloning and expression of two α-amylase genes from Streptococcus bovis 148 in Escherichia coli. Appl Environ Microbiol 59:3669–3673
Serror P, Sasaki T, Ehrlich SD, Maguin E (2002) Electrotransformation of Lactobacillus delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis with various plasmids. Appl Environ Microbiol 68:46–52
Skory CD (2003) Lactic acid production by Saccharomyces cerevisiae expressing a Rhizopus oryzae lactate dehydrogenase gene. J Ind Microbiol Biotechnol 30:22–27
Tanaka K, Komiyama A, Sonomoto K, Ishizaki A, Hall SJ, Stanbury PF (2002) Two different pathways for D-xylose metabolism and the effect of xylose concentration on the yield coefficient of L-lactate in mixed-acid fermentation by the lactic acid bacterium Lactococcus lactis IO-1. Appl Microbiol Biotechnol 60:160–167
Tarmy EM, Kaplan NO (1968) Kinetics of Escherichia coli B D-lactate dehydrogenase and evidence for pyruvate controlled change in conformation. J Biol Chem 243:2587–2596
Tateno T, Fukuda H, Kondo A (2007a) Production of L-lysine from starch by Corynebacterium glutamicum displaying α-amylase on its cell surface. Appl Microbiol Biotechnol 74:1213–1220
Tateno T, Fukuda H, Kondo A (2007b) Direct production of L-lysine from raw corn starch by Corynebacterium glutamicum secreting Streptococcus bovis α-amylase using cspB promoter and signal sequence. Appl Microbiol Biotechnol 77:533–541
Tokuhiro K, Ishida N, Kondo A, Takahashi H (2008) Lactic fermentation of cellobiose by a yeast strain displaying β-glucosidase on the cell surface. Appl Microbiol Biotechnol 79:481–488
Tokuhiro K, Ishida N, Nagamori E, Saitoh S, Onishi T, Kondo A, Takahashi H (2009) Double mutation of the PDC1 and ADH1 genes improves lactate production in the yeast Saccharomyces cerevisiae expressing the bovine lactate dehydrogenase gene. Appl Microbiol Biotechnol 82:883–890
Wee YJ, Kim JN, Ryu HW (2006) Biotechnological production of lactic acid and its recent applications. Food Technol Biotechnol 44:163–172
Wisselink HW, Toirkens MJ, del RF BM, Winkler AA, van Dijken JP, Pronk JT, van Maris AJA (2007) Engineering of Saccharomyces cerevisiae for efficient anaerobic alcoholic fermentation of L-arabinose. Appl Environ Microbiol 73:4881–4891
Yáñez R, Moldes AB, Alonso JL, Parajó JC (2003) Production of D(-)-lactic acid from cellulose by simultaneous saccharification and fermentation using Lactobacillus coryniformis subsp. torquens. Biotechnol Lett 25:1161–1164
Yoon HH (1997) Simultaneous saccharification and fermentation of cellulose for lactic acid production. Biotechnol Bioprocess Eng 2:101–104
Yukawa H, Omumasaba CA, Nonaka H, Kos P, Okai N, Suzuki N, Suda M, Tsuge Y, Watanabe J, Ikeda Y, Vertès AA, Inui M (2007) Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042–1058
Zhou S, Causey TB, Hasona A, Shanmugam KT, Ingram LO (2003a) Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69:399–407
Zhou S, Shanmugam KT, Ingram LO (2003b) Functional replacement of the Escherichia coli D-(-)-lactate dehydrogenase gene (ldhA) with the L-(+)-lactate dehydrogenase gene (ldhL) from Pediococcus acidilactici. Appl Environ Microbiol 69:2237–2244
Acknowledgements
This work was mainly supported by Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe), MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okano, K., Tanaka, T., Ogino, C. et al. Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85, 413–423 (2010). https://doi.org/10.1007/s00253-009-2280-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2280-5