Abstract
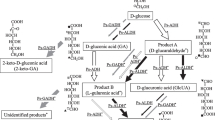
Welan gum is a microbial polysaccharide produced by Alcaligenes sp. CGMCC2428 that has d-glucose, d-glucuronic acid, d-glucose, and l-rhamnose as the main structural unit. The biosynthetic pathway of sugar nucleotides essential for producing welan gum in this strain was established in the following ways: (1) the detection of the presence of several intermediates and key enzymes; (2) the analysis of the response upon addition of precursors to the culture medium; (3) the correlation of the activities between several key enzymes with the yields of welan gum. With addition of 200-μM glucose-6-phosphate and fructose-6-phosphate, the production of welan gum was improved by 18%. The activities of phosphoglucomutase, phosphomannose isomerase, UDP-glucose pyrophosphorylase, and dTDP-glucose pyrophosphorylase, correlated well with the yields of welan gum. According to these findings, the biosynthetic pathway was proposed to involve the metabolism of glucose via two discrete systems. The first involves conversion of glucose to glucose-6-phosphate, with further reactions producing glucose-1-phosphate and fructose-6-phosphate, which are metabolized to the nucleotide sugar precursors of welan gum. The second system involves metabolism of glucose to synthesize the basic structural skeleton of the cell via central metabolic pathways, including the Entner–Doudoroff pathway, the pentose phosphate pathway, and the tricarboxylic acid cycle.





Similar content being viewed by others
References
Arrecubieta C, Garcia E, Lopez R (1996) Demonstration of UDP-glucose dehydrogenase activity in cell extracts of Escherichia coli expressing the pneumococcal cap3A gene required for the synthesis of type 3 capsular polysaccharide. J Bacteriol 178:2971–2974
Aw TY, Jones DP (1982) Direct determination of UDP-glucuronic acid in cell extracts by high-performance liquid chromatography. Anal Biochem 127:32–36
Basu SS, Dotson DD, Raetz CRH (2000) A facile enzymatic synthesis of uridine diphospho-[14C]galacturonic acid. Anal Biochem 280:173–177
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Chandrasekaran R, Radha A, Lee EJ (1994) Structural roles of calcium ions and side chains in welan: an X-ray study. Carbohydr Res 252:183–207
Duine JA, Jongejan JA (1989) Quinoproteins, enzymes with pyrrolo-quinoline quinone as cofactor. Annu Rev Biochem 58:403–426
Escalante A, Wacher RC, Garcoa GM, Farres A (1998) Enzymes involved in carbohydrate metabolism and their role on exopolysaccharide production in Streptococcus thermophilu. J Appl Microbiol 84:108–114
Fialho AM, Moreira LM, Granja AT, Hoffmann K, Popescu A, Sá-Correia I (2007) Biotechnology of the bacterial gellan gum: genes and enzymes of the biosynthetic pathway. In: Pereira MS (ed) A portrait of state-of-the-art research at the technical university of lisbon, 6th edn. Springer, Netherlands, pp 233–250
Goosen N, Vermaas DAM, Putte P (1987) Cloning of the genes involved in synthesis of coenzyme pyrrolo-quinoline-quinone from Acinetobacter calcoaceticus. J Bacteriol 169:303–307
Hsu CH, Lo YM (2003) Characterization of xanthan gum biosynthesis in a centrifugal, packed-bed reactor using metabolic flux analysis. Process Biochem 38:1617–1625
Kang KS, Veeder GT, Cottrell IW (1983) Some novel bacterial polysaccharides of recent development. Prog Ind Microbiol 18:231–253
Li M (2006) Effects of some single-gene knockout of the central metobolic enzymes on the metabolism in Escherichia coli. Dissertation, Zhejiang University
Li Y, He N, Guan H, Du G, Chen J (2003) A novel polygalacturonic acid bioflocculant REA-11 produced by Corynebacterium glutamicum: a proposed biosynthetic pathway and experimental confirmation. Appl Microbiol Biotechnol 63:200–206
Li S, Xu H, Shi N (2004) Production of a microbial polysaeeharides by fermentation. Food and Fermentation Industries 30:6–9
Lin TY, Hassid WZ (1966) Isolation of guanosine diphosphate uronic acids from a marine brown alga, Fucus gardneri Silva. J Biol Chem 241:3283–3293
Martins LO, Sá-Correia I (1991) Gellan gum biosynthetic enzymes in producing and nonproducing variants of Pseudomonas elodea. Biotechnol Appl Biochem 14:357–364
Ng FMW, Dawes EA (1973) Chemostat studies on the regulation of glucose metabolism in Pseudomonas aeruginosa by citrate. Biochem J 132:129–140
Nishioka T, Matsuda K, Fujita Y (2005) Combined analysis of metabolome and transcriptome: catabolism in bacillus subtilis. In: Tomita M (ed) Metabolomics—the frontier of systems biology, 9th edn. Springer, Tokyo, pp 127–140
O'Neill MA, Selvendran RR, Morris VJ, Eagles J (1986) Structure of the extracellular polysaccharide produced by the bacterium Alcaligenes (ATCC 31555) species. Carbohydr Res 145:295–313
Piggott NH, Sutherland IW, Jarman TR (1981) Enzymes involved in the biosynthesis of alginate by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 13:179–183
Pindar DF, Bucke C (1975) The biosynthesis of alginic acid by Azotobacter vinelandii. Biochem J 152:617–622
Pugashetti BK, Vadas L, Prihar HS, Feingold DS (1983) GDP-mannose dehydrogenase and biosynthesis of alginate-like polysaccharide in a mucoid strain of Pseudomonas aeruginosa. J Bacteriol 153:1107–1110
Rosalam S, England R (2006) Review of xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzyme Microb Technol 39:197–207
Sá-Correia I, Darzins A, Wang SK, Berry A, Chakrabarty AM (1987) Alginate biosynthetic enzymes in mucoid and nonmucoid Pseudomonas aeruginosa: overproduction of phosphomannose isomerase, phosphomannomutase, and GDP-mannose pyrophosphorylase by overexpression of the phosphomannose isomerase (pmi) gene. J Bacteriol 169:3224–3231
Sá-Correia I, Fialho AM, Videira P, Moreira LM, Marques AR, Albano H (2002) Gellan gum biosynthesis in Sphingomonas paucimobilis ATCC 31461: genes, enzymes and exopolysaccharide production engineering. J Ind Microbiol Biotechnol 29:170–176
Shuster CW, Doudoroff M (1967) Purification of 2-keto-3-deoxy-6-phosphohexonate aldolases of Pseudomonas saccharophila. Arch Microbiol 59:279–286
Silva E, Marques AR, Fialho AM, Granja AT, Sá-Correia I (2005) Proteins encoded by Sphingomonas elodea ATCC 31461 rmlA and ugpG genes, involved in gellan gum biosynthesis, exhibit both dTDP- and UDP-glucose pyrophosphorylase activities. Appl Environ Microbiol 71:4703–4712
Tejwani GA, Ramaiah A (1971) Properties of phsophofructokinase from the mucosa of rat jejunum and the relation to the lack of Pasteur effect. Biochem J 125:507–514
Vandamme EJ, Baets SD, Steinbüchel A (2002) Polysaccharides I: polysaccharides from prokaryotes. In: Vandamme EJ (ed) Biopolymers, 5th edn. Wiley-VCH, New York, pp 1–19
Videira PA, Cortes LL, Fialho AM, Sá-Correia I (2000) Identification of the pgmG gene, encoding a bifunctional protein with phosphoglucomutase and phosphomannomutase activities, in the gellan gum-producing strain Sphingomonas paucimobilis ATCC 31461. Appl Environ Microbiol 66:2252–2258
Whitfield C, Sutherland IW, Cripps RE (1982) Glucose metabolism in Xanthomonas campestris. J Gen Microbiol 128:981–985
Winkler HH (1973) Distribution of an inducible hexose-phosphate transport system among various bacteria. J Bacteriol 116(2):1079–1081
Zhu G, Li H (2004) Microbiology: science publishing company. Beijing, China
Acknowledgements
This work was supported by the National Basic Research Program of China (973; 2009CB724700), the National Nature Science Foundation of China (20674038, 20906050), and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 08KJA180001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Xu, H., Xu, H. et al. Biosynthetic pathway of sugar nucleotides essential for welan gum production in Alcaligenes sp. CGMCC2428. Appl Microbiol Biotechnol 86, 295–303 (2010). https://doi.org/10.1007/s00253-009-2298-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2298-8