Abstract
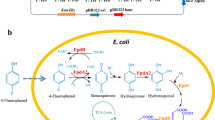
Escherichia coli cells, expressing 4-hydroxyphenylacetate 3-hydroxylase, fully transformed 4-halogenated phenols to their equivalent catechols as single products in shaken flasks. 4-Fluorophenol was transformed at a rate 1.6, 1.8, and 3.4-fold higher than the biotransformation of 4-chloro-, 4-bromo-, and 4-iodo-phenol, respectively. A scale-up from shaken flask to a 5 L stirred tank bioreactor was undertaken to develop a bioprocess for the production of 4-substituted halocatechols at higher concentrations and scale. In a stirred tank reactor, the optimized conditions for induction of 4-HPA hydroxylase expression were at 37 °C for 3 h. The rate of biotransformation of 4-fluorophenol to 4-fluorocatechol by stirred tank bioreactor grown cells was the same at 1 and 4.8 mM (5.13 μmol/min/g CDW) once the ratio of biocatalyst (E. coli CDW) to substrate concentration (mM) was maintained at 2:1. At 10.8 mM 4-fluorophenol, the rate of 4-fluorocatechol formation decreased by 4.7-fold. However, the complete transformation of 1.3 g of 4-fluorophenol (10.8 mM) to 4-fluorocatechol was achieved within 7 h in a 1 L reaction volume. Similar to 4-fluorophenol, other 4-substituted halophenols were completely transformed to 4-halocatechols at 2 mM within a 1–2 h period. An increase in 4-halophenol concentration to 4.8 mM resulted in a 2.5–20-fold decrease in biotransformation efficiency depending on the substrate tested. Organic solvent extraction of the 4-halocatechol products followed by column chromatography resulted in the production of purified products with a final yield of between 33% and 38%.

Similar content being viewed by others
References
Achkar J, Ferrandez A (2008) Fermentative production hydroxytyrosol using genetically engineered Escherichia coli. WO 2008/064839 A2 (DSM IP Assets B.V., Neth.), 42 pp
Alig L, Alsenz J, Andjelkovic M, Bendels S, Benardeau A, Bleicher K, Bourson A, David-Pierson P, Guba W, Hildbrand S, Kube D, Luebbers T, Mayweg AV, Narquizian R, Neidhart W, Nettekoven M, Plancher J-M, Rocha C, Rogers-Evans M, Roever S, Schneider G, Taylor S, Waldmeier P (2008) Benzodioxoles: novel cannabinoid-1 receptor inverse agonists for the treatment of obesity. J Med Chem 51:2115–2127
Allouche N, Damak M, Ellouz R, Sayadi S (2004) Use of whole cells of Pseudomonas aeruginosa for synthesis of the antioxidant hydroxytyrosol via conversion of tyrosol. Appl Environ Microbiol 70:2105–2109
Boshoff A, Burton MH, Burton SG (2003) Optimization of catechol production by membrane-immobilized polyphenol oxidase: a modeling approach. Biotechnol Bioeng 83:1–7
Brooks SJ, Doyle EM, Hewage C, Malthouse JPG, Duetz W, O’Connor KE (2004) Biotransformation of halophenols using crude cell extracts of Pseudomonas putida F6. Appl Microbiol Biotechnol 64:486–492
Bui VP, Hudlicky T, Hansen TV, Stenstrom Y (2002) Direct biooxidation of arenes to corresponding catechols with E. coli JM109 (pDTG602). Application to synthesis of combretastatins A-1 and B-1. Tetrahedron Lett 43:2839–2841
Claus H, Decker H (2006) Bacterial tyrosinases. Syst Appl Microbiol 29:3–14
Dixon S, Brown RCD, Gale PA (2007) A biaryl cross-coupling strategy for functionalization of benzocrown ethers. Chem Commun 34:3565–3567
Espin JC, Soler-Rivas C, Cantos E, Tomas-Barberan FA, Wichers HJ (2001) Synthesis of the antioxidant hydroxytyrosol using tyrosinase as biocatalyst. J Agric Food Chem 49:1187–1193
Fiege H, Voges H-W, Hamamoto T, Umemura S, Iwata T, Miki H, Fujita Y, Buysch H-J, Garbe D, Paulus W (2002) Phenol Derivatives. In: Wiley-VCH (ed) Ullmann’s Encyclopedia of Industrial Chemistry, vol 25. p 605
Hansen TV, Skattebol L (2005) One-pot synthesis of substituted catechols from the corresponding phenols. Tetrahedron Lett 46:3357–3358
Heinrich MR, Steglich W, Banwell MG, Kashman Y (2003) Total synthesis of the marine alkaloid halitulin. Tetrahedron 59:9239–9247
Held M, Suske W, Schmid A, Engesser K-H, Kohler H-PE, Witholt B, Wubbolts MG (1998) Preparative scale production of 3-substituted catechols using a novel monooxygenase from Pseudomonas azelaica HBP 1. J Mol Catal B: Enzym 5:87–93
Held M, Schmid A, Kohler H-P, Suske W, Witholt B, Wubbolts MG (1999) An integrated process for the production of toxic catechols from toxic phenols based on a designer biocatalyst. Biotechnol Bioeng 62:641–648
Hille UE, Hu Q, Vock C, Negri M, Bartels M, Mueller-Vieira U, Lauterbach T, Hartmann RW (2009) Novel CYP17 inhibitors: Synthesis, biological evaluation, structure-activity relationships and modeling of methoxy- and hydroxy-substituted methyleneimidazolyl biphenyls. Eur J Med Chem 44:2765–2775
Lee EY, Shuler ML (2007) Molecular engineering of epoxide hydrolase and its application to asymmetric and enantioconvergent hydrolysis. Biotechnol Bioeng 98:318–327
Lee J-Y, Xun L (1998) Novel biological process of l-DOPA production from l-tyrosine by p-hydroxyphenylacetate 3-hydroxylase. Biotechnol Lett 20:479–482
Liese A, Seelbach K, Wandrey C (2006) Industrial biotransformations, 2nd completely revised and extended edition. Wiley-VCH GmbH &Co. KGaA, Weinheim
Lisdat F, Wollenberger U (1998) Trienzyme amplification system for the detection of catechol and catecholamines using internal co-substrate regeneration. Anal Lett 31:1275–1285
Lynch RM, Woodley JM, Lilly MD (1997) Process design for the oxidation of fluorobenzene to fluorocatechol by Pseudomonas putida. J Biotechnol 58:167–175
Makrides SC (1996) Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev 60:512–538
Marin-Zamora ME, Rojas-Melgarejo F, Garcia-Canovas F, Garcia-Ruiz PA (2009) Production of o-diphenols by immobilized mushroom tyrosinase. J Biotechnol 139:163–168
Mason JR (1988) Oxygenase catalyzed hydroxylation of aromatic compounds: simple chemistry by complex enzymes. Int Ind Biotechnol 8:19–24
McClay K, Fox BG, Steffan RJ (2000) Toluene monooxygenase-catalyzed epoxidation of alkenes. Appl Environ Microbiol 66:1877–1882
Meyer A, Held M, Schmid A, Kohler H-PE, Witholt B (2003) Synthesis of 3-tert-butylcatechol by an engineered monooxygenase. Biotechnol Bioeng 81:518-524
Nolan LC, O’Connor KE (2007) Use of Pseudomonas mendocina, or recombinant Escherichia coli cells expressing toluene-4-monooxygenase, and a cell-free tyrosinase for the synthesis of 4-fluorocatechol from fluorobenzene. Biotechnol Lett 29:1045–1050
Paulini R, Lerner C, Diederich F, Jakob-Roetne R, Zurcher G, Borroni E (2006) Synthesis and biological evaluation of potent bisubstrate inhibitors of the enzyme catechol O-methyltransferase (COMT) lacking a nitro group. Helv Chim Acta 89:1856–1887
Pollard DJ, Woodley JM (2007) Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol 25:66–73
Prieto MA, Garcia JL (1994) Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli. A two-protein component enzyme. J Biol Chem 269:22823–22829
Prieto MA, Perez-Aranda A, Garcia JL (1993) Characterization of an Escherichia coli aromatic hydroxylase with a broad substrate range. J Bacteriol 175:2161–2167
Prieto MA, Diaz E, Garcia JL (1996) Molecular characterization of the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli W: engineering a mobile aromatic degradative cluster. J Bacteriol 178:111–120
Quideau S, Lebon M, Lamidey A-M (2002) Enantiospecific synthesis of the antituberculosis marine sponge metabolite (+)−puupehenone. The arenol oxidative activation route. Org Lett 4:3975–3978
Ridder L, Briganti F, Boersma MG, Boeren S, Vis EH, Scozzafava A, Veeger C, Rietjens IMCM (1998) Quantitative structure/activity relationship for the rate of conversion of C4-substituted catechols by catechol-1, 2-dioxygenase from Pseudomonas putida (arvilla) C1. Eur J Biochem 257:92–100
Rodriguez MJ, Lebrero JLA, Alvarez E (1999) Biotransformation of phenol to catechol by recombinant phenol hydroxylase. Biocatal Biotransform 17:45–60
Salvo JJ, Mobley DP, Brown DW, Caruso LA, Yake AP, Spivack JL, Dietrich DK (1990) Biosynthesis of p-hydroxylated aromatics. Biotechnol Prog 6:193–197
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature (London) 409:258–268
Selinheimo E, Gasparetti C, Mattinen M-L, Steffensen CL, Buchert J, Kruus K (2009) Comparison of substrate specificity of tyrosinases from Trichoderma reesei and Agaricus bisporus. Enzyme Microb Technol 44:1–10
Seo S-Y, Sharma VK, Sharma N (2003) Mushroom tyrosinase: recent prospects. J Agric Food Chem 51:2837–2853
Shadnia H, Wright JS (2008) Understanding the toxicity of phenols: using quantitative structure–activity relationship and enthalpy changes to discriminate between possible mechanisms. Chem Res Toxicol 21:1197–1204
Sonda S, Katayama K, Fujio M, Sakashita H, Inaba K, Asano K, Akira T (2007) 1, 5-Benzodioxepin derivatives as a novel class of muscarinic M3 receptor antagonists. Bioorg Med Chem Lett 17:925–931
Straathof AJJ, Panke S, Schmid A (2002) The production of fine chemicals by biotransformations. Curr Opin Biotechnol 13:548–556
Suske WA, Held M, Schmid A, Fleischmann T, Wubbolts MG, Kohler HP (1997) Purification and characterization of 2-hydroxybiphenyl 3-monooxygenase, a novel NADH-dependent, FAD-containing aromatic hydroxylase from Pseudomonas azelaica HBP1. J Biol Chem 272:24257–24265
Tao Y, Fishman A, Bentley WE, Wood TK (2004) Altering toluene 4-monooxygenase by active-site engineering for the synthesis of 3-methoxycatechol, methoxyhydroquinone, and methylhydroquinone. J Bacteriol 186:4705–4713
Ullrich R, Hofrichter M (2007) Enzymatic hydroxylation of aromatic compounds. Cell Mol Life Sci 64:271–293
Xie Q, Wu J, Xu G, Yang L (2006) Asymmetric reduction of o-chloroacetophenone with Candida pseudotropicalis 104. Biotechnol Prog 22:1301–1304
Yamaguchi S, Tsuchida N, Miyazawa M, Hirai Y (2005) Synthesis of two naturally occurring 3-methyl-2, 5-dihydro-1-benzoxepin carboxylic acids. J Org Chem 70:7505–7511
Acknowledgments
Lydie Coulombel was supported by Enterprise Ireland (PC/2008/131). Jasmina Nikodinovic was supported by the Environmental Protection Agency (2008-ET-LS-1-S2). Louise Nolan was supported by Science Foundation Ireland (04/IN3/B581).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coulombel, L., Nolan, L.C., Nikodinovic, J. et al. Biotransformation of 4-halophenols to 4-halocatechols using Escherichia coli expressing 4-hydroxyphenylacetate 3-hydroxylase. Appl Microbiol Biotechnol 89, 1867–1875 (2011). https://doi.org/10.1007/s00253-010-2969-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2969-5