Abstract
Purpose
Increased glycolytic activity on FDG PET/CT defines a subgroup of patients with metastatic gastroenteropancreatic neuroendocrine tumour (NET) with a poor prognosis. A limited range of systemic treatment options exist for more aggressive NET. The role of peptide receptor chemoradionuclide therapy (PRCRT) in such patients is, however, unclear. This retrospective study assessed the outcomes of patients with FDG-avid NET treated with PRCRT.
Methods
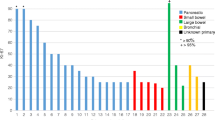
Clinical, biochemical and imaging response was assessed after completion of induction treatment of PRCRT with 5-fluorouracil in 52 patients selected for treatment on the basis of somatostatin-receptor imaging without spatially discordant FDG-avid disease. Of the cohort, 67 % had received prior chemotherapy. Overall survival (OS) and progression-free survival (PFS) were also analysed.
Results
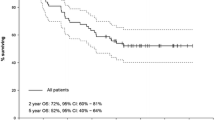
PRCRT was well tolerated with negligible grade 3/4 toxicities. After a median follow-up period of 36 months, the median OS was not achieved with a median PFS of 48 months. At 3 months after completion of PRCRT 2 % of patients showed a complete anatomical response, 28 % a partial response, 68 % stable disease, and only 2 % progression. On FDG PET/CT, 27 % achieved a complete metabolic response during the follow-up period. A biochemical response (>25 % fall in chromogranin-A levels) was seen in 45 %.
Conclusion
PRCRT is an effective treatment in patients with FDG-avid NET, even in patients who have failed conventional therapies. Given apparently higher response rates than with alternative therapeutic options and low toxicity, further research is needed to establish whether PRCRT should be used as a first-line treatment modality in this patient population.




Similar content being viewed by others
References
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12. doi:10.1097/MPA.0b013e3181ec124e.
Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60. doi:10.1093/annonc/mds276.
Oberg KE. The management of neuroendocrine tumours: current and future medical therapy options. Clin Oncol (R Coll Radiol). 2012;24:282–93. doi:10.1016/j.clon.2011.08.006.
Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76. doi:10.1159/000335597.
Hofman MS, Hicks RJ. Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med. 2012;14:71–81.
Adams S, Baum R, Rink T, Schumm-Drager PM, Usadel KH, Hor G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998;25:79–83.
Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–55. doi:10.1002/cncr.23469.
Pasquali C, Rubello D, Sperti C, Gasparoni P, Liessi G, Chierichetti F, et al. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg. 1998;22:588–92.
van Essen M, Sundin A, Krenning EP, Kwekkeboom DJ. Neuroendocrine tumours: the role of imaging for diagnosis and therapy. Nat Rev Endocrinol. 2014;10:102–14. doi:10.1038/nrendo.
Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–85. doi:10.1158/1078-0432.CCR-09-1759.
Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:302–11. doi:10.1007/s00259-010-1631-x.
Hubble D, Kong G, Michael M, Johnson V, Ramdave S, Hicks RJ. 177Lu-octreotate, alone or with radiosensitising chemotherapy, is safe in neuroendocrine tumour patients previously treated with high-activity 111In-octreotide. Eur J Nucl Med Mol Imaging. 2010;37:1869–75. doi:10.1007/s00259-010-1483-4.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA 0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. doi:10.1200/JCO.2007.15.2553.
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13. doi:10.1056/NEJMoa1003825.
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. doi:10.1056/NEJMoa1009290.
Kong G, Thompson M, Collins M, Herschtal A, Hofman MS, Johnston V. Assessment of predictors of response and long-term survival of patients with neuroendocrine tumour treated with peptide receptor chemoradionuclide therapy (PRCRT). Eur J Nucl Med Mol Imaging. 2014. doi:10.1007/s00259-014-2788-5.
Krenning EP, Valkema R, Kooij PP, Breeman WA, Bakker WH, de Herder WW. Scintigraphy and radionuclide therapy with [indium-111-labelled-diethyl triamine penta-acetic acid-D-Phe1]-octreotide. Ital J Gastroenterol Hepatol. 1999;31 Suppl 2:S219–223.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Krenning EP, Kooij PP, Pauwels S, Breeman WA, Postema PT, De Herder WW, et al. Somatostatin receptor: scintigraphy and radionuclide therapy. Digestion. 1996;57 Suppl 1:57–61.
Kong G, Johnston V, Ramdave S, Lau E, Rischin D, Hicks RJ. High-administered activity In-111 octreotide therapy with concomitant radiosensitizing 5FU chemotherapy for treatment of neuroendocrine tumors: preliminary experience. Cancer Biother Radiopharm. 2009;24:527–33. doi:10.1089/cbr.2009.0644.
Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27:561–9. doi:10.1089/cbr.2012.1276.
Cwikla JB, Sankowski A, Seklecka N, Buscombe JR, Nasierowska-Guttmejer A, Jeziorski KG, et al. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II study. Ann Oncol. 2010;21:787–94. doi:10.1093/annonc/mdp372.
Horsch D, Ezziddin S, Haug A, Gratz KF, Dunkelmann S, Krause BJ, et al. Peptide receptor radionuclide therapy for neuroendocrine tumors in Germany: first results of a multi-institutional cancer registry. Recent Results Cancer Res. 2013;194:457–65. doi:10.1007/978-3-642-27994-2_25.
Romer A, Seiler D, Marincek N, Brunner P, Koller MT, Ng QK, et al. Somatostatin-based radiopeptide therapy with [177Lu-DOTA]-TOC versus [90Y-DOTA]-TOC in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:214–22. doi:10.1007/s00259-013-2559-8.
Villard L, Romer A, Marincek N, Brunner P, Koller MT, Schindler C, et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol. 2012;30:1100–6. doi:10.1200/JCO.2011.37.2151.
Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–23. doi:10.1200/JCO.2010.33.7873.
van Essen M, Krenning EP, Kam BL, de Herder WW, van Aken MO, Kwekkeboom DJ. Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2008;35:743–8. doi:10.1007/s00259-007-0688-7.
Kesavan M, Claringbold PG, Turner JH. Hematological toxicity of combined Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology. 2014;99:108–17. doi:10.1159/000362558.
Binderup T, Knigge U, Loft A, Mortensen J, Pfeifer A, Federspiel B, et al. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J Nucl Med. 2010;51:704–12. doi:10.2967/jnumed.109.069765.
Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–8. doi:10.1200/JCO.2008.16.7858.
Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG; Eastern Cooperative Oncology Group. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23:4897–904.
Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–75. doi:10.1002/cncr.25425.
Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63. doi:10.1200/JCO.2009.22.8510.
Brizzi MP, Berruti A, Ferrero A, Milanesi E, Volante M, Castiglione F. Continuous 5-fluorouracil infusion plus long acting octreotide in advanced well-differentiated neuroendocrine carcinomas. A phase II trial of the Piemonte oncology network. BMC Cancer. 2009;9:388. doi:10.1186/1471-2407-9-388.
Delpassand ES, Samarghandi A, Mourtada JS, Zamanian S, Espenan GD, Sharif R, et al. Long-term survival, toxicity profile, and role of F-18 FDG PET/CT scan in patients with progressive neuroendocrine tumors following peptide receptor radionuclide therapy with high activity In-111 pentetreotide. Theranostics. 2012;2:472–80. doi:10.7150/thno.3739.
Yao JC, Pavel M, Phan AT, Kulke MH, Hoosen S, St Peter J, et al. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab. 2011;96:3741–9. doi:10.1210/jc.2011-0666.
Pape UF, Berndt U, Muller-Nordhorn J, Bohmig M, Roll S, Koch M, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocrinol Relat Cancer. 2008;15:1083–97. doi:10.1677/ERC-08-0017.
Barber TW, Hofman MS, Thomson BN, Hicks RJ. The potential for induction peptide receptor chemoradionuclide therapy to render inoperable pancreatic and duodenal neuroendocrine tumours resectable. Eur J Surg Oncol. 2012;38:64–71. doi:10.1016/j.ejso.2011.08.129.
Kipritidis J, Siva S, Hofman MS, Callahan J, Hicks RJ, Keall PJ. Validating and improving CT ventilation imaging by correlating with ventilation 4D-PET/CT using 68Ga-labeled nanoparticles. Med Phys. 2014;41:011910. doi:10.1118/1.4856055.
Stewart DJ, Kurzrock R. Fool’s gold, lost treasures, and the randomized clinical trial. BMC Cancer. 2013;13:193. doi:10.1186/1471-2407-13-193.
Conflicts of interest
None.
Funding
Professor Rodney Hicks was supported by a translational research grant of the Victorian Cancer Agency. Dr. K. Raghava Kashyap was supported by Endeavour Awards, a venture of Austraining International in partnership with the Department of Education, Employment and Workplace Relations, Government of Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. R. Kashyap and A/Prof M.S. Hofman are joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Overall survival of patients grouped based on (a) baseline CgA levels, (b) baseline performance status (ECOG), (c) Ki67 Grade and (d) FDG avidity (Grade 2–intensity of lesion being equal to liver, Grade 3–intensity of lesion being greater than liver, Grade 4–Standardized uptake value (SUVmax) more than 10) (GIF 22 kb)
Rights and permissions
About this article
Cite this article
Kashyap, R., Hofman, M.S., Michael, M. et al. Favourable outcomes of 177Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging 42, 176–185 (2015). https://doi.org/10.1007/s00259-014-2906-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2906-4