Abstract
Purpose
Niemann-Pick type C (NPC) is a cholesterol storage disease characterized by disruption in the endosomal–lysosomal transport system that leads to the accumulation of cholesterol and glycolipids in lysosomes. Developmental cognitive delay and progressive motor and cognitive impairment are characteristic of the disease. Tau accumulation has been reported in some NPC patients. We investigated the presence of tau and Aβ-amyloid deposits in a group of NPC patients and for comparison in age-matched healthy controls (HC).
Methods
Eight NPC patients and seven HC were included in the study. Participants underwent tau imaging with 18F-AV1451 and amyloid imaging with 11C-PiB. Both 18F-AV1451 and 11C-PiB standardized uptake value ratios were generated using the cerebellar cortex as the reference region. Associations between imaging results, and clinical and neurocognitive parameters were assessed through nonparametric analyses.
Results
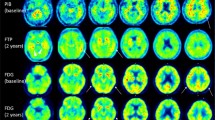
All participants were Aβ-negative. Four NPC patients presented with high tau burden in the brain. A 21-year-old female patient and a 40-year-old male patient showed high neocortical tau burden in a pattern different from that observed in patients with Alzheimer’s disease, while the same 40-year-old male patient, a 40-year-old female patient and a 50-year-old female patient showed high regional tau burden in the mesial temporal cortex. Spearman’s correlation analysis showed an association between tau burden in the mesial temporal lobe and age (p = 0.022), and age at symptom onset (p = 0.009), and between frontotemporal tau and duration of symptoms (p = 0.027). There were no correlations between global and regional tau and cognitive parameters.
Conclusion
Four of eight NPC patients showed tau deposition in the brain. The results of our exploratory study suggest that while tau deposits do not affect cognitive performance, tau deposits are associated with measures of disease onset and progression. Further studies in a larger cohort of NPC patients are needed to confirm these initial findings.


Similar content being viewed by others
References
Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277(5323):228–31.
Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, et al. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290(5500):2298–301. https://doi.org/10.1126/science.290.5500.2298.
Wassif CA, Cross JL, Iben J, Sanchez-Pulido L, Cougnoux A, Platt FM, et al. High incidence of unrecognized visceral/neurological late-onset Niemann-Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet Med. 2016;18(1):41–8. https://doi.org/10.1038/gim.2015.25.
Patterson MC, Hendriksz CJ, Walterfang M, Sedel F, Vanier MT, Wijburg F, et al. Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol Genet Metab. 2012;106(3):330–44. https://doi.org/10.1016/j.ymgme.2012.03.012.
Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685(1-3):48–62. https://doi.org/10.1016/j.bbalip.2004.08.011.
March P, Thrall M, Brown D, Mitchell T, Lowenthal A, Walkley S. GABAergic neuroaxonal dystrophy and other cytopathological alterations in feline Niemann-Pick disease type C. Acta Neuropathol. 1997;94:164–72.
Ong W, Kumar U, Switzer R, Sidhu A, Suresh G, Hu C, et al. Neurodegeneration in Niemann-Pick type C disease mice. Exp Brain Res. 2001;141:218–31.
Paul C, Boegle A, Maue R. Before the loss: neuronal dysfunction in Niemann-Pick type C disease. Biochim Biophys Acta. 2004;1685:63–76.
Fan Q-W, Yu W, Gong J-S, Zou K, Sawamura N, Senda T, et al. Cholesterol-dependent modulation of dendrite outgrowth and microtubule stability in cultured neurons. J Neurochem. 2002;80:178–90.
Sakamoto H, Ukena K, Tsutsu K. Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. J Neurosci. 2001;16:6221–32.
Walterfang M, Abel LA, Desmond P, Fahey MC, Bowman EA, Velakoulis D. Cerebellar volume correlates with saccadic gain and ataxia in adult Niemann-Pick type C. Mol Genet Metab. 2013;108(1):85–9. https://doi.org/10.1016/j.ymgme.2012.11.009.
Walterfang M, Fahey M, Desmond P, Wood A, Seal ML, Steward C, et al. White and gray matter alterations in adults with Niemann-Pick disease type C: a cross-sectional study. Neurology. 2010;75(1):49–56. https://doi.org/10.1212/WNL.0b013e3181e6210e.
Walterfang M, Patenaude B, Abel LA, Kluenemann H, Bowman EA, Fahey MC, et al. Subcortical volumetric reductions in adult Niemann-Pick disease type C: a cross-sectional study. AJNR Am J Neuroradiol. 2013;34(7):1334–40. https://doi.org/10.3174/ajnr.A3356.
Horoupian DS, Yang SS. Paired helical filaments in neurovisceral lipidosis (juvenile dystonic lipidosis). Ann Neurol. 1978;4(5):404–11. https://doi.org/10.1002/ana.410040504.
Auer IA, Schmidt ML, Lee VM, Curry B, Suzuki K, Shin RW, et al. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer's disease. Acta Neuropathol. 1995;90(6):547–51.
Love S, Bridges LR, Case CP. Neurofibrillary tangles in Niemann-Pick disease type C. Brain. 1995;118(Pt 1):119–29.
Suzuki K, Parker CC, Pentchev PG, Katz D, Ghetti B, D'Agostino AN, et al. Neurofibrillary tangles in Niemann-Pick disease type C. Acta Neuropathol. 1995;89(3):227–38.
Zhang M, Li J, Chakrabarty P, Bu B, Vincent I. Cyclin-dependent kinase inhibitors attenuate protein hyperphosphorylation, cytoskeletal lesion formation, and motor defects in Niemann-Pick type C mice. Am J Pathol. 2004;165(3):843–53.
Chiba Y, Komori H, Takei S, Hasegawa-Ishii S, Kawamura N, Adachi K, et al. Niemann-Pick disease type C1 predominantly involving the frontotemporal region, with cortical and brainstem Lewy bodies: an autopsy case. Neuropathology. 2014;34(1):49–57. https://doi.org/10.1111/neup.12047.
Bergeron D, Poulin S, Laforce R Jr. Cognition and anatomy of adult Niemann-Pick disease type C: insights for the Alzheimer field. Cogn Neuropsychol. 2018;35(3-4):209–22.
Bu B, Klunemann H, Suzuki K, Li J, Bird T, Jin LW, et al. Niemann-Pick disease type C yields possible clue for why cerebellar neurons do not form neurofibrillary tangles. Neurobiol Dis. 2002;11(2):285–97.
Villemagne VL, Doré V, Burnham SC, Masters CL, Rowe CC. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat Rev Neurol. 2018;14(4):225–36.
Walterfang M, Siu R, Velakoulis D. The NUCOG: validity and reliability of a brief cognitive screening tool in neuropsychiatric patients. Aust N Z J Psychiatry. 2006;40(11–12):995–1002. https://doi.org/10.1111/j.1440-1614.2006.01923.x.
Iturriaga C, Pineda M, Fernandez-Valero EM, Vanier MT, Coll MJ. Niemann-Pick C disease in Spain: clinical spectrum and development of a disability scale. J Neurol Sci. 2006;249(1):1–6. https://doi.org/10.1016/j.jns.2006.05.054.
Villemagne V, Dore V, Bourgeat P, Burnham S, Mulligan R, Laws S, et al. The Tau MeTeR composites for the generation of continuous and categorical measures of tau deposits in the brain. J Mol Med Ther. 2017;1(1):25–9.
Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18(4):351–7.
Ossenkoppele R, Schonhaut DR, Scholl M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139(Pt 5):1551–67. https://doi.org/10.1093/brain/aww027.
Marquie M, Siao Tick Chong M, Anton-Fernandez A, Verwer EE, Saez-Calveras N, Meltzer AC, et al. [F-18]-AV-1451 binding correlates with postmortem neurofibrillary tangle Braak staging. Acta Neuropathol. 2017;134(4):619–28. https://doi.org/10.1007/s00401-017-1740-8.
Scholl M, Ossenkoppele R, Strandberg O, Palmqvist S, Swedish Bio F, Jogi J, et al. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer's disease. Brain. 2017;140(9):2286–94. https://doi.org/10.1093/brain/awx171.
Zhang M, Wang X, Jiang F, Wang W, Vincent I, Bu B. Mitotic epitopes are incorporated into age-dependent neurofibrillary tangles in Niemann-Pick disease type C. Brain Pathol. 2010;20(2):367–77.
Ono M, Sahara N, Kumata K, Ji B, Ni R, Koga S, et al. Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegenerative tauopathies. Brain. 2017;140(3):764–80. https://doi.org/10.1093/brain/aww339.
Fan QW, Yu W, Senda T, Yanagisawa K, Michikawa M. Cholesterol-dependent modulation of tau phosphorylation in cultured neurons. J Neurochem. 2001;76(2):391–400.
Sawamura N, Gong JS, Chang TY, Yanagisawa K, Michikawa M. Promotion of tau phosphorylation by MAP kinase Erk1/2 is accompanied by reduced cholesterol level in detergent-insoluble membrane fraction in Niemann-Pick C1-deficient cells. J Neurochem. 2003;84(5):1086–96.
Distl R, Meske V, Ohm TG. Tangle-bearing neurons contain more free cholesterol than adjacent tangle-free neurons. Acta Neuropathol. 2001;101(6):547–54.
Pontecorvo MJ, Devous MD Sr, Navitsky M, Lu M, Salloway S, Schaerf FW, et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140(3):748–63. https://doi.org/10.1093/brain/aww334.
Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, et al. Region-specific association of subjective cognitive decline with tauopathy independent of global beta-amyloid burden. JAMA Neurol. 2017;74(12):1455–63.
Cho H, Chooi J, Lee S, Hwang M, Ryu Y, Lee M, et al. Tau burden and cognition in early-onset versus late-onset Alzheimer’s disease spectrum. Alzheimers Dement. 2016;12(7):S711–2.
Mattsson N, Zetterberg H, Bianconi S, Yanjanin NM, Fu R, Mansson JE, et al. Gamma-secretase-dependent amyloid-beta is increased in Niemann-Pick type C: a cross-sectional study. Neurology. 2011;76(4):366–72. https://doi.org/10.1212/WNL.0b013e318208f4ab.
Mattsson N, Zetterberg H, Bianconi S, Yanjanin NM, Fu R, Mansson JE, et al. Miglustat treatment may reduce cerebrospinal fluid levels of the axonal degeneration marker tau in Niemann-Pick type C. JIMD Rep. 2012;3:45–52. https://doi.org/10.1007/8904_2011_47.
Matsuo M, Shraishi K, Wada K, Ishitsuka Y, Doi H, Maeda M, et al. Effects of intracerebroventricular administration of 2-hydroxypropyl-beta-cyclodextrin in a patient with Niemann-Pick type C disease. Mol Genet Metab Rep. 2014;1:391–400. https://doi.org/10.1016/j.ymgmr.2014.08.004.
Bu B, Li J, Davies P, Vincent I. Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model. J Neurosci. 2002;22(15):6515–25.
Acknowledgments
We thank Avid Radiopharmaceuticals for providing AV1451 precursor and standard, especially Drs. Michael Pontecorvo and Michael Devous, for kindly providing 18F-AV1451 images of three young adults used for age-matched comparison with young NPC patients. We also thank Dr. Graeme O’Keefe, Dr. Gordon Chan, Dr. Kenneth Young, Dr. Sylvia Gong, Mrs. Denise El-Sheikh; and the Brain Research Institute for their assistance with this study.
Funding
This work was supported in part by the Austin Hospital Medical Research Foundation. The funding source was not involved in the study design, in the collection, analysis and interpretation of the data, in the writing of the report, or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
V.L.V. and M.W. conceived and designed the research. V.L.V., M.W., D.V., V.D., S.B., C.L.M. and C.C.R. performed the research and participated in the drafting of the work and revising it critically for important intellectual content. V.L.V. and M.W. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Villemagne, V.L., Velakoulis, D., Doré, V. et al. Imaging of tau deposits in adults with Niemann-Pick type C disease: a case-control study. Eur J Nucl Med Mol Imaging 46, 1132–1138 (2019). https://doi.org/10.1007/s00259-019-4273-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-4273-7