Abstract
Purpose
We aimed to investigate the role of FDG-PET/CT in monitoring of response and immune-related adverse events (irAEs) following first-line combination-immune checkpoint inhibitor (combination-ICI) therapy for advanced melanoma.
Methods
We retrospectively reviewed outcomes in patients who had (1) first-line nivolumab plus ipilimumab; (2) pre- and post-treatment FDG-PET/CT scans (pre-FDG-PET/CT and post-FDG-PET/CT) within 2 and 4 months of starting ICI, respectively; and (3) at least one lesion assessable by PET response criteria in solid tumors (PERCIST). Extracranial response was monitored by 3 monthly FDG-PET/CT. Whole-body metabolic tumor volume (wbMTV) was measured pre- and post-treatment and correlated with outcome. FDG-PET/CT manifestations of irAE were defined as new increased non-tumoral uptake on post-FDG-PET/CT and were correlated with clinical presentation.
Results
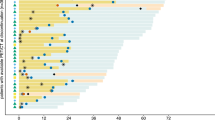
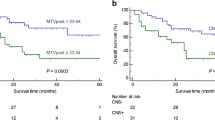
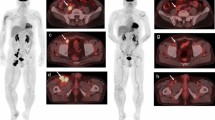
Thirty-one consecutive patients, median age 60 years (range, 30–78), were identified from 2016 to 2018. The median number of combination-ICI cycles to the first post-FDG-PET/CT response assessment was 3 (interquartile range (IQR), 2–4). The best-overall responses were complete metabolic response (CMR) in 25 (80%), partial metabolic response (PMR) in 3 (10%), and progressive metabolic disease (PMD) in 3 (10%) patients. Patients with PMD had significantly higher pre-treatment wbMTV (p = 0.009). At a median follow-up of 21.5 months, 26 (84%) patients were alive with median progression-free and overall survival not reached. Secondary progression occurred in 9/31 (29%) patients at a median of 8.2 months (IQR, 6.9–15.5), of those majority (78%) was detected by FDG-PET/CT. Of 36 findings on post-FDG-PET/CT suggestive of irAE, 29 (80%) had clinical confirmation. In 3 (7%), the FDG-PET/CT findings preceded clinical presentation. The most common FDG-PET/CT detectable irAEs were endocrinopathies (36%) and enterocolitis (35%).
Conclusion
FDG-PET/CT response evaluation predicts the long-term outcome of patients treated with first-line combination-ICIs. Long-term treatment response monitoring for detection of extracranial secondary progression is feasible by FDG-PET/CT. Beyond response assessment, FDG-PET/CT frequently detects clinically relevant irAEs, which may involve multiple systems contemporaneously or at various time-points and may precede clinical diagnosis.






Similar content being viewed by others
References
Wolchok JD, Rollin L, Larkin J. Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med. 2017;377:2503–4. https://doi.org/10.1056/NEJMc1714339.
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. https://doi.org/10.1056/NEJMoa1003466.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. https://doi.org/10.1056/NEJMoa1504030.
Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–92. https://doi.org/10.1016/S1470-2045(18)30700-9.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–46. https://doi.org/10.1056/NEJMoa1910836.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68. https://doi.org/10.1056/NEJMra1703481.
Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–86. https://doi.org/10.1038/nrclinonc.2016.58.
Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol. 2015;6:1. https://doi.org/10.3389/fimmu.2015.00001.
MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83. https://doi.org/10.1146/annurev-immunol-032712-095956.
Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–11. https://doi.org/10.1182/blood-2007-06-096297.
Wachsmann JW, Ganti R, Peng F. Immune-mediated disease in ipilimumab immunotherapy of melanoma with FDG PET-CT. Acad Radiol. 2017;24:111–5. https://doi.org/10.1016/j.acra.2016.08.005.
Nobashi T, Baratto L, Reddy SA, Srinivas S, Toriihara A, Hatami N, et al. Predicting response to immunotherapy by evaluating tumors, lymphoid cell-rich organs, and immune-related adverse events using FDG-PET/CT. Clin Nucl Med. 2019;44:e272–e9. https://doi.org/10.1097/RLU.0000000000002453.
Wong ANM, McArthur GA, Hofman MS, Hicks RJ. The advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur J Nucl Med Mol Imaging. 2017;44:67–77. https://doi.org/10.1007/s00259-017-3691-7.
Lewin J, Sayers L, Kee D, Walpole I, Sanelli A, Te Marvelde L, et al. Surveillance imaging with FDG-PET/CT in the post-operative follow-up of stage 3 melanoma. Ann Onc. 2018;29:1569–74. https://doi.org/10.1093/annonc/mdy124.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. https://doi.org/10.2967/jnumed.108.057307.
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. https://doi.org/10.1056/NEJMoa1414428.
Tan AC, Emmett L, Lo S, Liu V, Kapoor R, Carlino MS, et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Onc. 2018;29:2115–20. https://doi.org/10.1093/annonc/mdy330.
Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al. Novel patterns of response under immunotherapy. Ann Onc. 2019;30:385–96. https://doi.org/10.1093/annonc/mdz003.
Cho SY, Lipson EJ, Im HJ, Rowe SP, Gonzalez EM, Blackford A, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point (18)F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58:1421–8. https://doi.org/10.2967/jnumed.116.188839.
Sachpekidis C, Anwar H, Winkler J, Kopp-Schneider A, Larribere L, Haberkorn U, et al. The role of interim (18)F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2018;45:1289–96. https://doi.org/10.1007/s00259-018-3972-9.
Anwar H, Sachpekidis C, Winkler J, Kopp-Schneider A, Haberkorn U, Hassel JC, et al. Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging. 2018;45:376–83. https://doi.org/10.1007/s00259-017-3870-6.
Ito K, Teng R, Schoder H, Humm JL, Ni A, Michaud L, et al. (18)F-FDG PET/CT for monitoring of Ipilimumab therapy in patients with metastatic melanoma. J Nucl Med Med. 2019;60:335–41. https://doi.org/10.2967/jnumed.118.213652.
Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for assessing tumour response to immunotherapy: report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging. 2018. https://doi.org/10.1007/s00259-018-4171-4.
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–7. https://doi.org/10.1200/JCO.2015.64.0391.
Availability of data and material
The collected data is available for further analysis if required.
Code availability
Not applicable.
Funding
This research was partially supported by the Victorian Cancer Agency.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AI, MMS, AMW, RW, AG, AL, and MOH. The first draft of the manuscript was written by AI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Professor Rodney Hicks is the recipient of a National Health and Medical Research Foundation Practitioner Fellowship (APP1108050). All other authors declare no conflict of interest.
Ethics approval
This retrospective study was approved by Peter MacCallum Cancer Center Ethics Committee (approval number: 17/231R). Authors certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Consent to participate and consent to publication
Due to the retrospective nature of the study, informed consent was waived by the Peter MacCallum Cancer Center Ethics Committee (approval number: 17/231R). Ethics committee allowed the publication of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - General
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Iravani, A., Osman, M.M., Weppler, A.M. et al. FDG PET/CT for tumoral and systemic immune response monitoring of advanced melanoma during first-line combination ipilimumab and nivolumab treatment. Eur J Nucl Med Mol Imaging 47, 2776–2786 (2020). https://doi.org/10.1007/s00259-020-04815-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04815-w