Abstract
Purpose
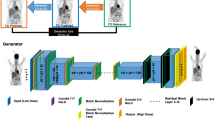
Estimation of accurate attenuation maps for whole-body positron emission tomography (PET) imaging in simultaneous PET-MRI systems is a challenging problem as it affects the quantitative nature of the modality. In this study, we aimed to improve the accuracy of estimated attenuation maps from MRI Dixon contrast images by training an augmented generative adversarial network (GANs) in a supervised manner. We augmented the GANs by perturbing the non-linear deformation field during image registration between MRI and the ground truth CT images.
Methods
We acquired the CT and the corresponding PET-MR images for a cohort of 28 prostate cancer patients. Data from 18 patients (2160 slices and later augmented to 270,000 slices) was used for training the GANs and others for validation. We calculated the error in bone and soft tissue regions for the AC μ-maps and the reconstructed PET images.
Results
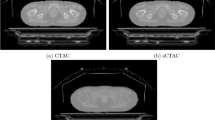
For quantitative analysis, we use the average relative absolute errors and validate the proposed technique on 10 patients. The DL-based MR methods generated the pseudo-CT AC μ-maps with an accuracy of 4.5% more than standard MR-based techniques. Particularly, the proposed method demonstrates improved accuracy in the pelvic regions without affecting the uptake values. The lowest error of the AC μ-map in the pelvic region was 1.9% for μ-mapGAN + aug compared with 6.4% for μ-mapdixon, 5.9% for μ-mapdixon + bone, 2.1% for μ-mapU-Net and 2.0% for μ-mapU-Net + aug. For the reconstructed PET images, the lowest error was 2.2% for PETGAN + aug compared with 10.3% for PETdixon, 8.7% for PETdixon + bone, 2.6% for PETU-Net and 2.4% for PETU-Net + aug..
Conclusion
The proposed technique to augment the training datasets for training of the GAN results in improved accuracy of the estimated μ-map and consequently the PET quantification compared to the state of the art.






Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hofmann M, Pichler B, Schölkopf B, Beyer T. Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques. Eur J Nucl Med Mol I. 2009;36:93–104.
Vandenberghe S, Marsden PK. PET-MRI: a review of challenges and solutions in the development of integrated multimodality imaging. Phys Med Biol. 2015;60:R115.
Chen Z, Jamadar SD, Li S, Sforazzini F, Baran J, Ferris N, et al. From simultaneous to synergistic MR-PET brain imaging: a review of hybrid MR-PET imaging methodologies. Hum Brain Mapp. 2018;39:5126–44.
Izquierdo-Garcia D, Catana C. MR imaging–guided attenuation correction of PET data in PET/MR imaging. PET Clinics. 2016;11:129–49.
Arabi H, Zaidi H. Magnetic resonance imaging-guided attenuation correction in whole-body PET/MRI using a sorted atlas approach. Med Image Anal. 2016;31:1–15.
Baran J, Chen Z, Sforazzini F, Ferris N, Jamadar S, Schmitt B, et al. Accurate hybrid template–based and MR-based attenuation correction using UTE images for simultaneous PET/MR brain imaging applications. BMC Med Imaging. 2018;18:41.
Izquierdo-Garcia D, Hansen AE, Förster S, Benoit D, Schachoff S, Fürst S, et al. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. J Nucl Med. 2014;55:1825–30.
Sekine T, Buck A, Delso G, Ter Voert EE, Huellner M, Veit-Haibach P, et al. Evaluation of atlas-based attenuation correction for integrated PET/MR in human brain: application of a head atlas and comparison to true CT-based attenuation correction. J Nucl Med. 2016;57:215–20.
Hofmann M, Bezrukov I, Mantlik F, Aschoff P, Steinke F, Beyer T, et al. MRI-based attenuation correction for whole-body PET/MRI: quantitative evaluation of segmentation-and atlas-based methods. J Nucl Med. 2011;52:1392–9.
Salomon A, Goedicke A, Schweizer B, Aach T, Schulz V. Simultaneous reconstruction of activity and attenuation for PET/MR. IEEE Trans Med Imaging. 2011;30:804–13.
Schick F. Whole-body MRI at high field: technical limits and clinical potential. Eur Radiol. 2005;15:946–59.
Gaertner FC, Fürst S, Schwaiger M. PET/MR: a paradigm shift. Cancer Imaging. 2013;13:36.
Lenzo N, Meyrick D, Turner J. Review of gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics. 2018;8:16.
Rhee H, Blazak J, Tham CM, Ng KL, Shepherd B, Lawson M, et al. Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016;6:76.
Greenspan H, Van Ginneken B, Summers RM. Guest editorial deep learning in medical imaging: overview and future promise of an exciting new technique. IEEE Trans Med Imaging. 2016;35:1153–9.
Li H. Deep learning for image denoising. Int J Signal Process Image Process Pattern Recognit. 2014;7:171–80.
Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS. Image reconstruction by domain-transform manifold learning. Nature. 2018;555:487.
Pawar K, Chen Z, Shah NJ, Egan GF. A deep learning framework for transforming image reconstruction into pixel classification. IEEE Access. 2019;7:177690–702.
Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for biomedical image segmentation. International Conference on Medical image computing and computer-assisted intervention: Springer; 2015. p. 234–41.
Isola P, Zhu J-Y, Zhou T, Efros AA. Image-to-image translation with conditional adversarial networks. Proceedings of the IEEE conference on computer vision and pattern recognition; 2017. p. 1125–34.
Goodfellow I, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S, et al. Generative adversarial nets. Advances in neural information processing systems; 2014. p. 2672–80.
Pathak D, Krahenbuhl P, Donahue J, Darrell T, Efros AA. Context encoders: feature learning by inpainting. Proceedings of the IEEE conference on computer vision and pattern recognition; 2016. p. 2536–44.
Zhang R, Isola P, Efros AA. Colorful image colorization. European conference on computer vision: Springer; 2016. p. 649–66.
Liu F, Jang H, Kijowski R, Bradshaw T, McMillan AB. Deep learning MR imaging–based attenuation correction for PET/MR imaging. Radiology. 2017;286:676–84.
Leynes AP, Yang J, Wiesinger F, Kaushik SS, Shanbhag DD, Seo Y, et al. Zero-echo-time and Dixon deep pseudo-CT (ZeDD CT): direct generation of pseudo-CT images for pelvic PET/MRI attenuation correction using deep convolutional neural networks with multiparametric MRI. J Nucl Med. 2018;59:852–8.
Torrado-Carvajal A, Vera-Olmos J, Izquierdo-Garcia D, Catalano OA, Morales MA, Margolin J, et al. Dixon-VIBE deep learning (DIVIDE) pseudo-CT synthesis for pelvis PET/MR attenuation correction. J Nucl Med. 2019;60:429–35.
Dong X, Wang T, Lei Y, Higgins K, Liu T, Curran WJ, et al. Synthetic CT generation from non-attenuation corrected PET images for whole-body PET imaging. Phys Med Biol. 2019;64:215016.
Varadhan R, Karangelis G, Krishnan K, Hui S. A framework for deformable image registration validation in radiotherapy clinical applications. J Appl Clin Med Phys. 2013;14:192–213.
Keszei AP, Berkels B, Deserno TM. Survey of non-rigid registration tools in medicine. J Digit Imaging. 2017;30:102–16. https://doi.org/10.1007/s10278-016-9915-8.
Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS). Insight J. 2009;2:1–35.
Carney JP, Townsend DW, Rappoport V, Bendriem B. Method for transforming CT images for attenuation correction in PET/CT imaging. Med Phys. 2006;33:976–83. https://doi.org/10.1118/1.2174132.
Han JH, Yang S, Lee BU. A novel 3-D color histogram equalization method with uniform 1-D gray scale histogram. IEEE Trans Image Process. 2011;20:506–12. https://doi.org/10.1109/tip.2010.2068555.
Ioffe S, Szegedy C. Batch normalization: accelerating deep network training by reducing internal covariate shift. arXiv preprint arXiv:150203167. 2015.
Goscinski WJ, McIntosh P, Felzmann UC, Maksimenko A, Hall CJ, Gureyev T, et al. The multi-modal Australian ScienceS Imaging and Visualization Environment (MASSIVE) high performance computing infrastructure: applications in neuroscience and neuroinformatics research. Front Neuroinform. 2014;8:30.
Kinehan P, Fletcher J. PET/CT standardized uptake values (SUVs) in clinical practice and assessing response to therapy. Semin Ultrasound CT MR. 2010;31:496–505.
Shen D, Wu G, Suk H-I. Deep learning in medical image analysis. Annu Rev Biomed Eng. 2017;19:221–48.
Lundervold AS, Lundervold A. An overview of deep learning in medical imaging focusing on MRI. Z Med Phys. 2019;29:102–27.
Lei Y, Fu Y, Wang T, Qiu RL, Curran WJ, Liu T, et al. Deep learning in multi-organ segmentation. arXiv preprint arXiv:200110619. 2020.
Hesamian MH, Jia W, He X, Kennedy P. Deep learning techniques for medical image segmentation: achievements and challenges. J Digit Imaging. 2019;32:582–96.
Fu Y, Lei Y, Wang T, Curran WJ, Liu T, Yang X. Deep learning in medical image registration: A Review. arXiv preprint arXiv:191212318. 2019.
Pawar K, Chen Z, Shah NJ, Egan GF. Suppressing motion artefacts in MRI using an Inception-ResNet network with motion simulation augmentation. NMR in Biomedicine. 2019:e4225.
Lv J, Yang M, Zhang J, Wang X. Respiratory motion correction for free-breathing 3D abdominal MRI using CNN-based image registration: a feasibility study. Br J Radiol. 2018;91:20170788.
Lee H, Yune S, Mansouri M, Kim M, Tajmir SH, Guerrier CE, et al. An explainable deep-learning algorithm for the detection of acute intracranial haemorrhage from small datasets. Nat Biomed Eng. 2019;3:173.
Acknowledgements
The authors acknowledge Richard McIntyre from Monash Biomedical Imaging for their assistance in acquiring data and Keiran O’Brien and Daniel Staeb from Siemens Healthineers for useful discussions.
Funding
The research was supported by a grant from the Reignwood Cultural Foundation and an Australian Research Council (ARC) Linkage grant (LP170100494) that includes financial support from Siemens Healthineers. GE is supported by the ARC Centre of Excellence for Integrative Brain Function (CE140100007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Monash University Human Research Ethical Committee.
Informed consent
Informed consent from all individual participants in this study was obtained by Dr. Jeremy Grummet.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Technology
Rights and permissions
About this article
Cite this article
Pozaruk, A., Pawar, K., Li, S. et al. Augmented deep learning model for improved quantitative accuracy of MR-based PET attenuation correction in PSMA PET-MRI prostate imaging. Eur J Nucl Med Mol Imaging 48, 9–20 (2021). https://doi.org/10.1007/s00259-020-04816-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04816-9