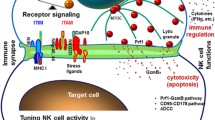
Abstract
Natural killer (NK) cells represent a promising cell type to utilize for effective adoptive immunotherapy. However, little is known about the important cytolytic molecules and signaling pathways used by NK cells in the adoptive transfer setting. To address this issue, we developed a novel mouse model to investigate the trafficking and mechanism of action of these cells. We demonstrate that methylcholanthrene-induced RKIK sarcoma cells were susceptible to NK cell-mediated lysis in vitro and in vivo following adoptive transfer of NK cells in C57BL/6 RAG-2−/−γc−/− mice. Cytotoxic molecules perforin, granzymes B and M as well as the death ligand TRAIL and pro-inflammatory cytokine IFN-γ were found to be important in the anti-tumor effect mediated by adoptively transferred NK cells. Importantly, we demonstrate that adoptively transferred NK cells could traffic to the tumor site and persisted in vivo which correlated with the anti-tumor effect observed. Overall, the results of this study have important implications for enhancing NK cell-based immunotherapies.








Similar content being viewed by others
References
Yokoyama WM, Kim S, French AR (2004) The dynamic life of natural killer cells. Annu Rev Immunol 22:405–429
Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23:225–274
Cavanaugh VJ, Raulet DH, Campbell AE (2007) Upregulation of CD94/NKG2A receptors and Qa-1b ligand during murine cytomegalovirus infection of salivary glands. J Gen Virol 88:1440–1445
Gasser S, Orsulic S, Brown EJ, Raulet DH (2005) The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436:1186–1190
Raulet DH, Guerra N (2009) Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol 9:568–580
Smyth MJ, Thia KY, Cretney E et al (1999) Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol 162:6658–6662
Seki N, Hayakawa Y, Brooks AD et al (2003) Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res 63:207–213
Wu J, Lanier LL (2003) Natural killer cells and cancer. Adv Cancer Res 90:127–156
Smyth MJ, Crowe NY, Godfrey DI (2001) NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol 13:459–463
Degli-Esposti MA, Smyth MJ (2005) Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol 5:112–124
Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y (2004) Cytokines in cancer immunity and immunotherapy. Immunol Rev 202:275–293
Rosenberg S (1985) Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J Natl Cancer Inst 75:595–603
Bordignon C, Carlo-Stella C, Colombo MP et al (1999) Cell therapy: achievements and perspectives. Haematologica 84:1110–1149
Morecki S, Yacovlev E, Gelfand Y, Vilensky A, Slavin S (2004) Allogeneic versus syngeneic killer splenocytes as effector cells for the induction of graft-versus-tumor effect. Biol Blood Marrow Transplant 10:40–48
Kawase T, Matsuo K, Kashiwase K et al (2009) HLA mismatch combinations associated with decreased risk of relapse: implications for the molecular mechanism. Blood 113:2851–2858
Ruggeri L, Capanni M, Urbani E et al (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295:2097–2100
Guven H, Gilljam M, Chambers BJ et al (2003) Expansion of natural killer (NK) and natural killer-like T (NKT)-cell populations derived from patients with B-chronic lymphocytic leukemia (B-CLL): a potential source for cellular immunotherapy. Leukemia 17:1973–1980
Alici E, Sutlu T, Bjorkstrand B et al (2008) Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood 111:3155–3162
Imai C, Iwamoto S, Campana D (2005) Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106:376–383
Cho D, Campana D (2009) Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med 29:89–96
Karre K, Ljunggren HG, Piontek G, Kiessling R (1986) Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319:675–678
Pegram HJ, Jackson JT, Smyth MJ, Kershaw MH, Darcy PK (2008) Adoptive transfer of gene-modified primary NK cells can specifically inhibit tumor progression in vivo. J Immunol 181:3449–3455
Abdool K, Cretney E, Brooks AD et al (2006) NK cells use NKG2D to recognize a mouse renal cancer (Renca), yet require intercellular adhesion molecule-1 expression on the tumor cells for optimal perforin-dependent effector function. J Immunol 177:2575–2583
Takeda K, Smyth MJ, Cretney E et al (2002) Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med 195:161–169
Trapani JA, Smyth MJ (2002) Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol 2:735–747
Screpanti V, Wallin RP, Ljunggren HG, Grandien A (2001) A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J Immunol 167:2068–2073
Trapani JA (2001) Granzymes: a family of lymphocyte granule serine proteases. Genome Biol 2:3014 (reviews)
Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ (2002) Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol 168:1356–1361
Nagata S, Suda T (1995) Fas and Fas ligand: lpr and gld mutations. Immunol Today 16:39–43
Rabinowich H, Vitolo D, Altarac S, Herberman RB, Whiteside TL (1992) Role of cytokines in the adoptive immunotherapy of an experimental model of human head and neck cancer by human IL-2-activated natural killer cells. J Immunol 149:340–349
Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75:163–189
Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y (2005) NKG2D function protects the host from tumor initiation. J Exp Med 202:583–588
Huang J, Khong HT, Dudley ME et al (2005) Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother 28:258–267
Zhou J, Dudley ME, Rosenberg SA, Robbins PF (2005) Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother 28:53–62
Dudley ME, Wunderlich JR, Robbins PF et al (2002) Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298:850–854
Besser MJ, Shapira-Frommer R, Treves AJ et al (2009) Minimally cultured or selected autologous tumor-infiltrating lymphocytes after a lympho-depleting chemotherapy regimen in metastatic melanoma patients. J Immunother 32:415–423
Lakshmikanth T, Burke S, Ali TH et al (2009) NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest 119:1251–1263
van den Broek MF, Kagi D, Zinkernagel RM, Hengartner H (1995) Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur J Immunol 25:3514–3516
Street SE, Cretney E, Smyth MJ (2001) Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 97:192–197
Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ (2007) Apoptosis induced by the lymphocyte effector molecule perforin. Curr Opin Immunol 19:339–347
Keefe D, Shi L, Feske S et al (2005) Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity 23:249–262
Metkar SS, Wang B, Aguilar-Santelises M et al (2002) Cytotoxic cell granule-mediated apoptosis: perforin delivers granzyme B-serglycin complexes into target cells without plasma membrane pore formation. Immunity 16:417–428
Sarin A, Williams MS, Alexander-Miller MA, Berzofsky JA, Zacharchuk CM, Henkart PA (1997) Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity 6:209–215
Darmon AJ, Nicholson DW, Bleackley RC (1995) Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature 377:446–448
Waterhouse NJ, Sedelies KA, Trapani JA (2006) Role of Bid-induced mitochondrial outer membrane permeabilization in granzyme B-induced apoptosis. Immunol Cell Biol 84:72–78
Kelly JM, Waterhouse NJ, Cretney E et al (2004) Granzyme M mediates a novel form of perforin-dependent cell death. J Biol Chem 279:22236–22242
Beresford PJ, Xia Z, Greenberg AH, Lieberman J (1999) Granzyme A loading induces rapid cytolysis and a novel form of DNA damage independently of caspase activation. Immunity 10:585–594
Ebnet K, Hausmann M, Lehmann-Grube F et al (1995) Granzyme A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J 14:4230–4239
Trapani JA, Bird PI (2008) A renaissance in understanding the multiple and diverse functions of granzymes? Immunity 29:665–667
Buzza MS, Zamurs L, Sun J et al (2005) Extracellular matrix remodeling by human granzyme B via cleavage of vitronectin, fibronectin, and laminin. J Biol Chem 280:23549–23558
Metkar SS, Menaa C, Pardo J et al (2008) Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity 29:720–733
Robbins PF, Dudley ME, Wunderlich J et al (2004) Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol 173:7125–7130
Cooper MA, Bush JE, Fehniger TA et al (2002) In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 100:3633–3638
Prlic M, Blazar BR, Farrar MA, Jameson SC (2003) In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med 197:967–976
Becknell B, Caligiuri MA (2005) Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol 86:209–239
Hayakawa Y, Smyth MJ (2006) CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 176:1517–1524
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22:633–640
Colucci F, Caligiuri MA, Di Santo JP (2003) What does it take to make a natural killer? Nat Rev Immunol 3:413–425
Klingemann HG, Martinson J (2004) Ex vivo expansion of natural killer cells for clinical applications. Cytotherapy 6:15–22
Fujisaki H, Kakuda H, Shimasaki N et al (2009) Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 69:4010–4017
Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R (2009) Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood 113:6120–6127
Roda JM, Parihar R, Carson WE 3rd (2005) CpG-containing oligodeoxynucleotides act through TLR9 to enhance the NK cell cytokine response to antibody-coated tumor cells. J Immunol 175:1619–1627
Muller T, Uherek C, Maki G et al (2008) Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother 57:411–423
Acknowledgments
We would like to acknowledge the assistance of the Peter MacCallum Cancer Centre Experimental Animal Facility technicians for animal care, in particular Michelle Stirling and Leanne McNiff for maintenance of the gene-targeted mice used in this study. We would also like to acknowledge Nicole McLaughlin for generating some of the antibodies used in these experiments. This work was funded by project grants from the National Health and Medical Research Council (NHMRC), Cancer Council of Victoria and the Susan Komen Breast Cancer Foundation. M.H. Kershaw and P.K. Darcy were supported by a NHMRC Senior Research Fellowship and Career Development Award, respectively. M.J. Smyth was supported by a NHMRC Senior Principal Research Fellowship. N.M. Haynes was supported by a NHMRC CJ Martin Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. H. Kershaw and P. K. Darcy contributed equally as senior authors.
Rights and permissions
About this article
Cite this article
Pegram, H.J., Haynes, N.M., Smyth, M.J. et al. Characterizing the anti-tumor function of adoptively transferred NK cells in vivo. Cancer Immunol Immunother 59, 1235–1246 (2010). https://doi.org/10.1007/s00262-010-0848-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0848-7