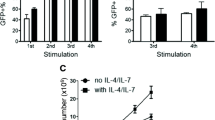
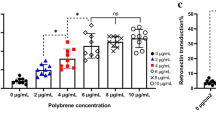
Abstract
The anti-tumor efficacy of TCR-engineered T cells in vivo depends largely on less-differentiated subsets such as T cells with naïve-like T cell (TN) phenotypes with greater expansion and long-term persistence. To increase these subsets, we compared the generation of New York esophageal squamous cell carcinoma-1 (NY-ESO-1)-specific T cells under supplementation with either IL-2 or IL-7/IL-15. PBMCs were transduced with MS3II-NY-ESO-1-siTCR retroviral vector. T cell generation was adapted from a CD19-specific CART cell production protocol. Comparable results in viability, expansion and transduction efficiency of T cells under stimulation with either IL-2 or IL-7/IL-15 were observed. IL-7/IL-15 led to an increase of CD4+ T cells and a decrease of CD8+ T cells, enriched the amount of TN among CD4+ T cells but not among CD8+ T cells. In a 51Cr release assay, similar specific lysis of NY-ESO-1-positive SW982 sarcoma cells was achieved. However, intracellular cytokine staining revealed a significantly increased production of IFN-γ and TNF-α in T cells generated by IL-2 stimulation. To validate these unexpected findings, NY-ESO-1-specific T cell production was evaluated in another protocol originally established for TCR-engineered T cells. IL-7/IL-15 increased the proportion of TN. However, the absolute number of TN did not increase due to a significantly slower expansion of T cells with IL-7/IL-15. In conclusion, IL-7/IL-15 does not seem to be superior to IL-2 for the generation of NY-ESO-1-specific T cells. This is in sharp contrast to the observations in CD19-specific CART cells. Changes of cytokine cocktails should be carefully evaluated for individual vector systems.






Similar content being viewed by others
Abbreviations
- 51Cr:
-
Chromium-51
- ACD-A:
-
Anticoagulant citrate dextrose solution
- ACT:
-
Adoptive cell therapy
- HDs:
-
Healthy donors
- NY-ESO-1:
-
New York esophageal squamous cell carcinoma-1
- TCM :
-
Central memory-like T cell(s)
- TE :
-
Effector-like T cell(s)
- TEM :
-
Effector memory-like T cell(s)
- Th cells:
-
T helper cells
- TN :
-
Naïve-like T cell(s)
- TSCM :
-
Stem cell memory T cell(s)
References
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348(6230):62–68. https://doi.org/10.1126/science.aaa4967
Kunert A, Straetemans T, Govers C, Lamers C, Mathijssen R, Sleijfer S, Debets R (2013) TCR-engineered T cells meet new challenges to treat solid tumors: choice of antigen, T cell fitness, and sensitization of tumor milieu. Front Immunol 4:363
Park TS, Groh EM, Patel K, Kerkar SP, Lee CC, Rosenberg SA (2016) Expression of MAGE-A and NY-ESO-1 in primary and metastatic cancers. J Immunother 39(1):1–7. https://doi.org/10.1097/cji.0000000000000101
Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J (2006) Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res 12(3 Pt 1):764–771. https://doi.org/10.1158/1078-0432.Ccr-05-1544
Endo M, de Graaff MA, Ingram DR, Lim S, Lev DC, Briaire-de Bruijn IH, Somaiah N, Bovee JV, Lazar AJ, Nielsen TO (2015) NY-ESO-1 (CTAG1B) expression in mesenchymal tumors. Mod Pathol 28(4):587–595. https://doi.org/10.1038/modpathol.2014.155
Lai JP, Robbins PF, Raffeld M, Aung PP, Tsokos M, Rosenberg SA, Miettinen MM, Lee CC (2012) NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol 25(6):854–858. https://doi.org/10.1038/modpathol.2012.31
Schmitt M, Huckelhoven AG, Hundemer M, Schmitt A, Lipp S, Emde M, Salwender H, Hanel M, Weisel K, Bertsch U, Durig J, Ho AD, Blau IW, Goldschmidt H, Seckinger A, Hose D (2017) Frequency of expression and generation of T-cell responses against antigens on multiple myeloma cells in patients included in the GMMG-MM5 trial. Oncotarget 8(49):84847–84862. https://doi.org/10.18632/oncotarget.11215
van Rhee F, Szmania SM, Zhan F, Gupta SK, Pomtree M, Lin P, Batchu RB, Moreno A, Spagnoli G, Shaughnessy J, Tricot G (2005) NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood 105(10):3939–3944. https://doi.org/10.1182/blood-2004-09-3707
Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL (2011) Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29(7):917
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA (2006) Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314(5796):126–129. https://doi.org/10.1126/science.1129003
Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Li YF, El-Gamil M, Rosenberg SA (2015) A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 21(5):1019–1027. https://doi.org/10.1158/1078-0432.Ccr-14-2708
D’Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, Grupp S, Tap WD, Chagin K, Binder GK, Basu S, Lowther DE, Wang R, Bath N, Tipping A, Betts G, Ramachandran I, Navenot JM, Zhang H, Wells DK, Van Winkle E, Kari G, Trivedi T, Holdich T, Pandite L, Amado R, Mackall CL (2018) Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 (c259)T cells in synovial sarcoma. Cancer Discov 8(8):944–957. https://doi.org/10.1158/2159-8290.Cd-17-1417
Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J, Kulikovskaya I, Sinha SK, Kronsberg S, Gupta M, Bond S, Melchiori L, Brewer JE, Bennett AD, Gerry AB, Pumphrey NJ, Williams D, Tayton-Martin HK, Ribeiro L, Holdich T, Yanovich S, Hardy N, Yared J, Kerr N, Philip S, Westphal S, Siegel DL, Levine BL, Jakobsen BK, Kalos M, June CH (2015) NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 21(8):914–921. https://doi.org/10.1038/nm.3910
Thomas R, Al-Khadairi G, Roelands J, Hendrickx W, Dermime S, Bedognetti D, Decock J (2018) NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol 9:947. https://doi.org/10.3389/fimmu.2018.00947
Woloszynska-Read A, Mhawech-Fauceglia P, Yu J, Odunsi K, Karpf AR (2008) Intertumor and intratumor NY-ESO-1 expression heterogeneity is associated with promoter-specific and global DNA methylation status in ovarian cancer. Clin Cancer Res 14(11):3283–3290. https://doi.org/10.1158/1078-0432.Ccr-07-5279
Ochi T, Fujiwara H, Okamoto S, An J, Nagai K, Shirakata T, Mineno J, Kuzushima K, Shiku H, Yasukawa M (2011) Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood 118(6):1495–1503. https://doi.org/10.1182/blood-2011-02-337089
Provasi E, Genovese P, Lombardo A, Magnani Z, Liu PQ, Reik A, Chu V, Paschon DE, Zhang L, Kuball J, Camisa B, Bondanza A, Casorati G, Ponzoni M, Ciceri F, Bordignon C, Greenberg PD, Holmes MC, Gregory PD, Naldini L, Bonini C (2012) Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med 18(5):807–815. https://doi.org/10.1038/nm.2700
Schmid DA, Irving MB, Posevitz V, Hebeisen M, Posevitz-Fejfar A, Sarria JC, Gomez-Eerland R, Thome M, Schumacher TN, Romero P, Speiser DE, Zoete V, Michielin O, Rufer N (2010) Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J Immunol 184(9):4936–4946. https://doi.org/10.4049/jimmunol.1000173
Govers C, Sebestyen Z, Roszik J, van Brakel M, Berrevoets C, Szoor A, Panoutsopoulou K, Broertjes M, Van T, Vereb G, Szollosi J, Debets R (2014) TCRs genetically linked to CD28 and CD3epsilon do not mispair with endogenous TCR chains and mediate enhanced T cell persistence and anti-melanoma activity. J Immunol 193(10):5315–5326. https://doi.org/10.4049/jimmunol.1302074
Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, Palmer DC, Ji Y, Reger RN, Leonard WJ, Danner RL, Rosenberg SA, Restifo NP (2009) Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA 106(41):17469–17474. https://doi.org/10.1073/pnas.0907448106
Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C (2011) A human memory T cell subset with stem cell-like properties. Nat Med 17(10):1290
Klebanoff CA, Scott CD, Leonardi AJ, Yamamoto TN, Cruz AC, Ouyang C, Ramaswamy M, Roychoudhuri R, Ji Y, Eil RL, Sukumar M, Crompton JG, Palmer DC, Borman ZA, Clever D, Thomas SK, Patel S, Yu Z, Muranski P, Liu H, Wang E, Marincola FM, Gros A, Gattinoni L, Rosenberg SA, Siegel RM, Restifo NP (2016) Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Invest 126(1):318–334. https://doi.org/10.1172/jci81217
Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu J, Kaech SM (2015) IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell 161(4):750–761. https://doi.org/10.1016/j.cell.2015.03.021
Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F, Lupo-Stanghellini MT, Mavilio F, Mondino A, Bicciato S, Recchia A, Bonini C (2013) IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121(4):573–584. https://doi.org/10.1182/blood-2012-05-431718
Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, Liu H, Creighton CJ, Gee AP, Heslop HE, Rooney CM, Savoldo B, Dotti G (2014) Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123(24):3750–3759. https://doi.org/10.1182/blood-2014-01-552174
Kondo T, Imura Y, Chikuma S, Hibino S, Omata-Mise S, Ando M, Akanuma T, Iizuka M, Sakai R, Morita R, Yoshimura A (2018) Generation and application of human induced-stem cell memory T cells for adoptive immunotherapy. Cancer Sci 109(7):2130–2140. https://doi.org/10.1111/cas.13648
Hoffmann J-M, Schubert M-L, Wang L, Hückelhoven A, Sellner L, Stock S, Schmitt A, Kleist C, Gern U, Loskog A (2018) Differences in expansion potential of naive chimeric antigen receptor T cells from healthy donors and untreated chronic lymphocytic leukemia Patients. Front Immunol 8:1956
Stock S, Hoffmann J-M, Schubert M-L, Wang L, Wang S, Gong W, Neuber B, Gern U, Schmitt A, Müller-Tidow C (2018) Influence of retronectin-mediated T-cell activation on expansion and phenotype of CD19-specific chimeric antigen receptor T cells. Hum Gene Ther 29(10):1167–1182
Stock S, Ubelhart R, Schubert ML, Fan F, He B, Hoffmann JM, Wang L, Wang S, Gong W, Neuber B, Huckelhoven-Krauss A, Gern U, Christ C, Hexel M, Schmitt A, Schmidt P, Krauss J, Jager D, Muller-Tidow C, Dreger P, Schmitt M, Sellner L (2019) Idelalisib for optimized CD19-specific chimeric antigen receptor T cells in chronic lymphocytic leukemia patients. Int J Cancer. https://doi.org/10.1002/ijc.32201
Kaartinen T, Luostarinen A, Maliniemi P, Keto J, Arvas M, Belt H, Koponen J, Loskog A, Mustjoki S, Porkka K (2017) Low interleukin-2 concentration favors generation of early memory T cells over effector phenotypes during chimeric antigen receptor T-cell expansion. Cytotherapy 19(6):689–702
Mercier-Letondal P, Montcuquet N, Sauce D, Certoux JM, Jeanningros S, Ferrand C, Bonyhadi M, Tiberghien P, Robinet E (2008) Alloreactivity of ex vivo-expanded T cells is correlated with expansion and CD4/CD8 ratio. Cytotherapy 10(3):275–288. https://doi.org/10.1080/14653240801927032
Gargett T, Brown MP (2015) Different cytokine and stimulation conditions influence the expansion and immune phenotype of third-generation chimeric antigen receptor T cells specific for tumor antigen GD2. Cytotherapy 17(4):487–495
Yu S, Nukaya I, Enoki T, Chatani E, Kato A, Goto Y, Dan K, Sasaki M, Tomita K, Tanabe M (2008) In vivo persistence of genetically modified T cells generated ex vivo using the fibronectin CH296 stimulation method. Cancer Gene Ther 15(8):508–516
Yang S, Ji Y, Gattinoni L, Zhang L, Yu Z, Restifo NP, Rosenberg SA, Morgan RA (2013) Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol Immunother 62(4):727–736
Yang S, Archer GE, Flores CE, Mitchell DA, Sampson JH (2013) A cytokine cocktail directly modulates the phenotype of DC-enriched anti-tumor T cells to convey potent anti-tumor activities in a murine model. Cancer Immunol Immunother 62(11):1649–1662
Xu XJ, Song DG, Poussin M, Ye Q, Sharma P, Rodríguez-García A, Tang Y-M, Powell DJ (2016) Multiparameter comparative analysis reveals differential impacts of various cytokines on CART cell phenotype and function ex vivo and in vivo. Oncotarget 7(50):82354–82368
Kayser S, Bobeta C, Feucht J, Witte KE, Scheu A, Bulow HJ, Joachim S, Stevanovic S, Schumm M, Rittig SM, Lang P, Rocken M, Handgretinger R, Feuchtinger T (2015) Rapid generation of NY-ESO-1-specific CD4(+) THELPER1 cells for adoptive T-cell therapy. Oncoimmunology 4(5):e1002723. https://doi.org/10.1080/2162402x.2014.1002723
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207(10):2187–2194. https://doi.org/10.1084/jem.20100643
Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM (2010) Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 207(10):2175–2186. https://doi.org/10.1084/jem.20100637
Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM (2012) IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest 122(4):1271–1282. https://doi.org/10.1172/jci59806
Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR (2011) Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 117(17):4501–4510. https://doi.org/10.1182/blood-2010-10-310425
Mikucki ME, Skitzki JJ, Frelinger JG, Odunsi K, Gajewski TF, Luster AD, Evans SS (2016) Unlocking tumor vascular barriers with CXCR42: implications for cancer immunotherapy. Oncoimmunology 5(5):e1116675. https://doi.org/10.1080/2162402x.2015.1116675
Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Frelinger JG, Odunsi K, Gajewski TF, Luster AD, Evans SS (2015) Non-redundant requirement for CXCR43 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun 6:7458. https://doi.org/10.1038/ncomms8458
Chheda ZS, Sharma RK, Jala VR, Luster AD, Haribabu B (2016) Chemoattractant receptors BLT1 and CXCR44 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors. J Immunol 197(5):2016–2026. https://doi.org/10.4049/jimmunol.1502376
Schuster K, Gadiot J, Andreesen R, Mackensen A, Gajewski TF, Blank C (2009) Homeostatic proliferation of naive CD8+ T cells depends on CD62L/L-selectin-mediated homing to peripheral LN. Eur J Immunol 39(11):2981–2990. https://doi.org/10.1002/eji.200939330
Gattinoni L, Klebanoff CA, Restifo NP (2012) Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer 12(10):671–684. https://doi.org/10.1038/nrc3322
Farber DL, Yudanin NA, Restifo NP (2014) Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol 14(1):24–35. https://doi.org/10.1038/nri3567
Funding
This work was funded in part by the Government of Baden-Württemberg (Anschubfinanzierung zur Etablierung eines Netzwerks “Brückeninstitutionen für die Regenerative Medizin in Baden-Württemberg”, Kapitel 1403 Tit.Gr. 74), by the DKTK (Deutsches Konsortium für Translationale Krebsforschung) and by the NCT-HD-CAR-1 Grant from the German Cancer Research Center (DKFZ). Leopold Sellner was supported by the “Physician Scientist-Programm” of the Medical Faculty of Heidelberg, the NCT Heidelberg School of Oncology (HSO) and the “Clinician Scientist Programm” of the German Society of Internal Medicine (DGIM).
Author information
Authors and Affiliations
Contributions
WG and LS designed the study; WG, JMH and YL performed experiments; WG and LS analyzed the data and wrote the manuscript; MS edited the manuscript; MS, LW, MLS, WG, SS, BN, AHK, UG and AS discussed the experimental design; CMT and HS read the manuscript and gave comments; HS provided essential materials; all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval and ethical standards
The research was approved by the Ethics Committee of the University of Heidelberg (S-254/2016). All studies involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent for the use of their blood for research purposes was obtained from all healthy donors by the Blood Bank Heidelberg.
Cell line authentication
The soft-tissue sarcoma cell lines SW982 (HLA-A2-positive NY-ESO-1-positive), SYO-1 (HLA-A2-negative NY-ESO-1-negative), Fuji (HLA-A2-positive NY-ESO-1-negative) and MLS-1765-92 (HLA-A2-negative NY-ESO-1-positive) were provided by Prof. H. Shiku (Mie University, Tsu, Japan). All cell lines were authenticated at DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gong, W., Hoffmann, JM., Stock, S. et al. Comparison of IL-2 vs IL-7/IL-15 for the generation of NY-ESO-1-specific T cells. Cancer Immunol Immunother 68, 1195–1209 (2019). https://doi.org/10.1007/s00262-019-02354-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02354-4