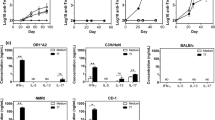
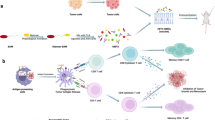
Abstract
Cancer is one of the main causes of mortality worldwide and a major public health concern. Among various strategies, therapeutic vaccines have been developed to stimulate anti-tumoral immune responses. However, in spite of extensive studies, this approach suffers from a lack of efficacy. Recently, we designed the MAG-Tn3 vaccine, aiming to induce antibody responses against Tn, a tumor-associated carbohydrate antigen. The Tn antigen is of interest because it is expressed by several adenocarcinomas, but not normal cells. The fully synthetic glycopeptide vaccine MAG-Tn3 is composed of four arms built on three adjacent Tn moieties associated with the tetanus toxin-derived peptide TT830–844 CD4+ T-cell epitope. This promiscuous CD4+ T-cell epitope can bind to a wide range of HLA–DRB molecules and is thus expected to activate CD4+ T-cell responses in a large part of the human population. The MAG-Tn3 vaccine was formulated with the GSK-proprietary immunostimulant AS15, composed of CpG7909, MPL, and QS21, which has been shown to stimulate both innate and humoral responses, in addition to being well tolerated. Here, seven patients with localized breast cancer with a high-risk of relapse were immunized with the MAG-Tn3 vaccine formulated with AS15. The first results of phase I clinical trial demonstrated that all vaccinated patients developed high levels of Tn-specific antibodies. Moreover, these antibodies specifically recognized Tn-expressing human tumor cells and killed them through a complement-dependent cytotoxicity mechanism. Overall, this study establishes, for the first time, the capacity of a fully synthetic glycopeptide cancer vaccine to induce specific immune responses in humans.




Similar content being viewed by others
References
Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P (2017) Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol 38:577–593. https://doi.org/10.1016/j.it.2017.05.006
Ahmed MS, Bae YS (2014) Dendritic cell-based therapeutic cancer vaccines: past, present and future. Clin Exp Vaccine Res 3:113–116. https://doi.org/10.7774/cevr.2014.3.2.113
Kantoff PW, Higano CS, Shore ND et al (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422. https://doi.org/10.1056/NEJMoa1001294
Vermaelen K (2019) Vaccine strategies to improve anti-cancer cellular immune responses. Front Immunol 10:8. https://doi.org/10.3389/fimmu.2019.00008
Dillman RO (2017) Is there a role for therapeutic cancer vaccines in the age of checkpoint inhibitors? Hum Vaccines Immunother 13:528–532. https://doi.org/10.1080/21645515.2016.1244149
Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, Thielemans K, Neyns B (2016) Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol 34:1330–1338. https://doi.org/10.1200/JCO.2015.63.4121
Gibney GT, Kudchadkar RR, DeConti RC et al (2015) Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res 21:712–720. https://doi.org/10.1158/1078-0432.CCR-14-2468
Ott PA, Wu CJ (2019) Cancer vaccines: steering T cells down the right path to eradicate tumors. Cancer Discov. https://doi.org/10.1158/2159-8290.cd-18-1357
Hollingsworth RE, Jansen K (2019) Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 4:7. https://doi.org/10.1038/s41541-019-0103-y
Fu C, Zhao H, Wang Y, Cai H, Xiao Y, Zeng Y, Chen H (2016) Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA 88:275–286. https://doi.org/10.1111/tan.12900
Cheever MA, Allison JP, Ferris AS et al (2009) The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res 15:5323–5337. https://doi.org/10.1158/1078-0432.CCR-09-0737
Mazal D, Lo-Man R, Bay S et al (2013) Monoclonal antibodies toward different Tn-amino acid backbones display distinct recognition patterns on human cancer cells. Implications for effective immuno-targeting of cancer. Cancer Immunol Immunother 62:1107–1122. https://doi.org/10.1007/s00262-013-1425-7
Desai PR (2000) Immunoreactive T and Tn antigens in malignancy: role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus Med Rev 14:312–325. https://doi.org/10.1053/tmrv.2000.16229
Springer GF (1984) T and Tn, general carcinoma autoantigens. Science 224:1198–1206
Springer GF (1997) Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med 75:594–602
Radhakrishnan P, Dabelsteen S, Madsen FB et al (2014) Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci USA 111:E4066–E4075. https://doi.org/10.1073/pnas.1406619111
Loureiro LR, Carrascal MA, Barbas A, Ramalho JS, Novo C, Delannoy P, Videira PA (2015) Challenges in antibody development against Tn and sialyl-Tn antigens. Biomolecules 5:1783–1809. https://doi.org/10.3390/biom5031783
Slovin SF, Ragupathi G, Musselli C et al (2003) Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol 21:2489–4292. https://doi.org/10.1200/JCO.2003.04.112
Scheid E, Major P, Bergeron A et al (2016) Tn-MUC1 DC vaccination of rhesus macaques and a phase I/II trial in patients with nonmetastatic castrate-resistant prostate cancer. Cancer Immunol Res 4:881–892. https://doi.org/10.1158/2326-6066.CIR-15-0189
Skwarczynski M, Toth I (2016) Peptide-based synthetic vaccines. Chem Sci 7:842–854. https://doi.org/10.1039/c5sc03892h
Ganneau C, Simenel C, Emptas E et al (2017) Large-scale synthesis and structural analysis of a synthetic glycopeptide dendrimer as an anti-cancer vaccine candidate. Org Biomol Chem. https://doi.org/10.1039/c6ob01931e
Lo-Man R, Vichier-Guerre S, Perraut R et al (2004) A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res 64:4987–4994. https://doi.org/10.1158/0008-5472.CAN-04-0252
Laubreton D, Bay S, Sedlik C et al (2016) The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830–844 universal epitope provides anti-tumor immunity. Cancer Immunol Immunother 65:315–325. https://doi.org/10.1007/s00262-016-1802-0
Dreno B, Thompson JF, Smithers BM et al (2018) MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 19:916–929. https://doi.org/10.1016/s1470-2045(18)30254-7
Lo-Man R, Vichier-Guerre S, Bay S, Dériaud E, Cantacuzène D, Leclerc C (2001) Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. J Immunol 166:2849–2854
Bay S, Lo-Man R, Osinaga E, Nakada H, Leclerc C, Cantacuzène D (1997) Preparation of a multiple antigen glycopeptide (MAG) carrying the Tn antigen. A possible approach to a synthetic carbohydrate vaccine. J Pept Res 49:620–625. https://doi.org/10.1111/j.1399-3011.1997.tb01171.x
Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A (1989) Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol 19:2237–2242. https://doi.org/10.1002/eji.1830191209
Nakada H, Inoue M, Tanaka N, Numata Y, Kitagawa H, Fukui S, Yamashina I (1991) Expression of the Tn antigen on T-lymphoid cell line Jurkat. Biochem Biophys Res Commun 179:762–767. https://doi.org/10.1016/0006-291X(91)91882-D
Tarp MA, Clausen H (2008) Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta 1780:546–563. https://doi.org/10.1016/j.bbagen.2007.09.010
Coelho H, Matsushita T, Artigas G et al (2015) The quest for anticancer vaccines: deciphering the fine-epitope specificity of cancer-related monoclonal antibodies by combining microarray screening and saturation transfer difference NMR. J Am Chem Soc 137:12438–12441. https://doi.org/10.1021/jacs.5b06787
Slovin SF, Ragupathi G, Fernandez C et al (2007) A polyvalent vaccine for high-risk prostate patients: “are more antigens better?”. Cancer Immunol Immunother 56:1921–1930. https://doi.org/10.1007/s00262-007-0335-y
Freire T, Zhang X, Dériaud E et al (2010) Glycosidic Tn-based vaccines targeting dermal dendritic cells favor germinal center B-cell development and potent antibody response in the absence of adjuvant. Blood 116:3526–3536. https://doi.org/10.1182/blood-2010-04-279133
Kruit WH, Suciu S, Dreno B et al (2013) Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol 31:2413–2420. https://doi.org/10.1200/JCO.2012.43.7111
Bouche FB, Steinmetz A, Yanagi Y, Muller CP (2005) Induction of broadly neutralizing antibodies against measles virus mutants using a polyepitope vaccine strategy. Vaccine 23:2074–2077. https://doi.org/10.1016/j.vaccine.2005.01.011
Yu Z, Healy F, Valmori D, Escobar P, Corradin G, Mach JP (1994) Peptide-antibody conjugates for tumour therapy: a MHC-class-II-restricted tetanus toxin peptide coupled to an anti-Ig light chain antibody can induce cytotoxic lysis of a human B-cell lymphoma by specific CD4 T cells. Int J Cancer 56:244–248
James EA, Bui J, Berger D, Huston L, Roti M, Kwok WW (2007) Tetramer-guided epitope mapping reveals broad, individualized repertoires of tetanus toxin-specific CD4+ T cells and suggests HLA-based differences in epitope recognition. Int Immunol 19:1291–1301. https://doi.org/10.1093/intimm/dxm099
Deng N, Weaver JM, Mosmann TR (2014) Cytokine diversity in the Th1-dominated human anti-influenza response caused by variable cytokine expression by Th1 cells, and a minor population of uncommitted IL-2+IFNgamma-Thpp cells. PLoS ONE 9:e95986. https://doi.org/10.1371/journal.pone.0095986
Mamidi S, Höne S, Kirschfink M (2017) The complement system in cancer: ambivalence between tumour destruction and promotion. Immunobiology 222:45–54. https://doi.org/10.1016/j.imbio.2015.11.008
Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS (2015) Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol 67:171–182. https://doi.org/10.1016/j.molimm.2015.03.255
Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M (2009) Specificity and affinity of human Fc receptors and their polymorphic variants for human IgG subclasses. Blood 113:3716–3725. https://doi.org/10.1182/blood-2008-09-179754
Holmberg LA, Sandmaier BM (2004) Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev Vaccines 3:655–663. https://doi.org/10.1586/14760584.3.6.655
Afshar-Kharghan V (2017) The role of the complement system in cancer. J Clin Investig 127:780–789. https://doi.org/10.1172/JCI90962
Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, Botto M, Introna M, Golay J (2014) Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol 171:1581–1587. https://doi.org/10.4049/jimmunol.171.3.1581
Bologna L, Gotti E, Da Roit F, Intermesoli T, Rambaldi A, Introna M, Golay J (2012) Ofatumumab is more efficient than rituximab in lysing B chronic lymphocytic leukemia cells in whole blood and in combination with chemotherapy. J Immunol 190:231–239. https://doi.org/10.4049/jimmunol.1202645
Wang SY, Veeramani S, Racila E, Cagley J, Fritzinger DC, Vogel CW, St John W, Weiner GJ (2009) Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood 114:5322–5330. https://doi.org/10.1182/blood-2009-01-200469
Posey AD, Schwab RD, Boesteanu AC et al (2016) Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44:1444–1454. https://doi.org/10.1016/j.immuni.2016.05.014
Joyce JA, Fearon DT (2015) T cell exclusion, immune privilege, and the tumor microenvironment. Science 348:74–80. https://doi.org/10.1126/science.aaa6204
van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM (2017) Migrating into the tumor: a roadmap for T cells. Trends Cancer 3:797–808. https://doi.org/10.1016/j.trecan.2017.09.006
Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, Behrens MD, Knutson KL (2014) Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res 74:2974–2985. https://doi.org/10.1158/0008-5472.CAN-13-2564
Acknowledgements
We thank Jamila Louahed (GSK Vaccines) for her continuous support, helpful discussions and critical review of the publication and the clinical protocol. We thank the CB UTechS platform and the molecular biophysics facility (especially Sylviane Hoos) for their support and help with the various devices and Luisa Nardini, Natalia Zmarlak, Ferdinand Nanfack Minkeu, and Inge Holm for sharing equipment. We thank Eleonore De Guillebon from the Hôpital Européen Georges Pompidou and Thomas Bachelot from the Centre Hospitalier Léon Bérard for their valuable support in the organization of this clinical trial. We thank the hospital staff for their daily work on the project and the patients for their agreement to participate in the trial.
Funding
The work was funded by the donors of the MAG-Tn3 program. GlaxoSmithKline Biological SA provided AS15 and preparing the final formulation of the MAG/AS15 vaccine.
Author information
Authors and Affiliations
Contributions
CL conceived and supervised the study, discussed the data, and wrote the manuscript. PR designed and performed the experiments analyzed the results and wrote the manuscript. CA coordinated the clinical trial, participated in the collection of the clinical data, and provided input for the manuscript. SB developed the process of vaccine synthesis and provided input for the manuscript. CG prepared the Tn3 antigen used in the ELISA. MC (investigator coordinator), SD, JM, CG, DL, FP, M-PS, OT, and AV designed and performed oversight of the clinical trial.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rosenbaum, P., Artaud, C., Bay, S. et al. The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients. Cancer Immunol Immunother 69, 703–716 (2020). https://doi.org/10.1007/s00262-020-02503-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02503-0