Abstract
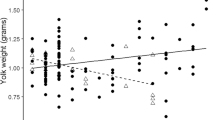
In some bird species, mothers can advantage the offspring of one sex either by elevating them in the laying order to promote earlier hatching or by allocating greater resources to eggs of the preferred sex. In size dimorphic species, the predictions as to which sex should benefit most from such pre-laying adjustments are ambiguous. The smaller sex would benefit from an initial size advantage to help compensate for the faster growth rate of the larger sex. However, an early advantage to offspring of the larger sex might have a greater effect on their lifetime reproductive success than an equivalent advantage to offspring of the smaller sex. We investigated these hypotheses in the polygynous brown songlark, Cinclorhamphus cruralis, which is one of the most sexually size dimorphic birds known. We conducted within-clutch comparisons and found that females hatched from larger eggs and were initially heavier (but not structurally larger) than their brothers. This may afford females an early competitive advantage, as egg volume remained correlated with chick mass until at least 5 days of age. Similarly, we found that hatch order was still positively associated with nestling mass and size when the brood was 10 days of age, but there was no clear relationship between offspring sex and hatching order. During this study, food was plentiful and there were few obvious cases of nestling starvation. When food is limited, we suggest that the greater nutrient reserves of female hatchlings could not only help compensate for their slower growth, but could also give them a survival advantage over their brothers early in the nestling period. Consequently, egg size dimorphism may be an adaptation that facilitates an early shift in brood sex-ratio towards cheaper daughters in conditions of low food availability.



Similar content being viewed by others
References
Albrecht DJ (2000) Sex ratio manipulation within broods of house wrens, Troglodytes aedon. Anim Behav 59:1227–1234
Amundsen T, Stokland JM (1990) Egg size and parental quality influence nestling growth in the Shag. Auk 10:410–413
Anderson DJ, Reeve J, Martinez Gomez JE, Weathers WW, Hutson S, Cunningham HV, Bird DM (1993) Sexual size dimorphism and food requirements of nestling birds. Can J Zool 71:2541–2545
Anderson DJ, Reeve JD, Bird DM (1997) Sexually dimorphic eggs, nestling growth and sibling competition in American kestrels, Falco sparverius. Funct Ecol 11:331–335
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, N.J.
Ankney CD (1980) Egg weight, survival and growth of Lesser Snow Goose goslings. J Wildl Manage 44:174–182
Badyaev AV, Hill GE, Beck ML, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA (2002) Sex-biased hatching order and adaptive population divergence in a passerine bird. Science 295:316–318
Bednarz JC, Hayden TJ (1991) Skewed brood sex ratio and sex-biased hatching sequence in Harris's hawks. Am Nat 137:116–132
Blakers M, Davies SJJF, Reilly PN (1984) The atlas of Australian Birds. Royal Australian Ornithologists Union and Melbourne University Press, Melbourne
Blanco G, Davila JA, Septiem JAL, Rodriguez R, Martinez F (2002) Sex-biased initial eggs favour sons in the slightly size-dimorphic Scops owl (Otus scops). Biol J Linn Soc 76:1–7
Bortolotti GR (1986) Influence of sibling competition on nestling sex ratios of sexually dimorphic birds. Am Nat 127:495–507
Both C, Visser ME, Verboven N (1999) Density-dependent recruitment rates in great tits: the importance of being heavier. Proc R Soc Lond B 266:465–469
Charnov EL (1982) The Theory of Sex Allocation. Princeton University Press, Princeton
Clotfelter ED (1996) Mechanisms of facultative sex ratio variation in zebra finches (Taeniopygia guttata). Auk 113:441-449
Cordero PJ, Griffiths SC, Aparicio JM, Parkin DT (2000) Sexual dimorphism in house sparrow eggs. Behav Ecol Sociobiol 48:353–357
Cordero PJ, Vinuela J, Aparicio JM, Veiga JP (2001) Seasonal variation in sex ratio and sexual egg dimorphism favouring daughters in first clutches of the spotless starling. J Evol Biol 14:829–834
Frank SA (1990) Sex allocation theory for birds and mammals. Annu Rev Ecol Syst. 21:13–55
Griffiths R (1992) Sex-biased mortality in the lesser black-backed gull Larus fuscus during the nestling stage. Ibis 134:237–244
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Howe HF (1976) Egg size, hatching asynchrony, sex, and brood reduction in the Common Grackle. Ecology 57:1195–1207
Hoyt DF (1979) Practical methods of estimating volume and fresh weight of bird eggs. Auk 96:73–77
Johnson AL (1986) Reproduction in the female. In: Sturkie PD (ed) Avian physiology. Springer, Berlin Heidelberg New York, pp 403–431.
Kilner R (1998) Primary and secondary sex ratio manipulation by Zebra Finches. Anim Behav 56:155–164
Komdeur J, Magrath MJL, Krackow S (2002) Pre-ovulation control of hatching sex ratio in the Seychelles warbler. Proc R Soc Lond B 269:1067–1072
Krackow S (1999) Avian sex ratio distortions: the myth of maternal control. In: Adams N, Slotov N (eds) Proc Int Ornithol Congr 22:425–433
Krebs EA, Green DJ, Double MC, Griffiths R (2002) Laying date and laying sequence influences the sex ratio of crimson rosella broods. Behav Ecol Sociobiol 51:447–454
Krijgsveld KL, Dijkstra C, Daan S (1998) Energy requirements for growth in relation to sexual size dimorphism in marsh harrier, Circus aeroginosus, nestlings. Physiol Zool 71:693–702
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lahiri D, Bye S, Nurnberger J, Hodes M, Crisp M (1992) A non-organic and non-enzymatic extraction method gives higher yields of genomic DNA from whole-blood samples than do nine other methods tested. J Biochem Biophys Methods 25:193–205
Legge S, Heinsohn R, Double, MC, Grifiths R, Cockburn A (2001) Complex sex allocation in the laughing kookaburra. Behav Ecol 12:524–533
Magrath RD (1991) Nestling weight and juvenile survival in the blackbird, Turdus merula. J Anim Ecol 60:335–351
Magrath RD (1992) The effect of egg mass on the growth and survival of Blackbirds: a field experiment. J Zool 227:639–653
Manly BJF (1997) Randomization, bootstrap and Monte Carlo methods in biology. Chapman and Hall, London
Mead PS, Morton ML, Fish BE (1987) Sexual dimorphism in egg size and implications regarding facultative manipulation of sex in mountain white-crowned sparrows. Condor 89:798–803
Monros JS, Belda EJ, Barba E (2002) Post-fledging survival of individual great tits: the effect of hatching date and fledging mass. Oikos 99:481–488
Myers JH (1978) Sex ratio adjustment under food stress: maximization of quality or numbers of offspring? Am Nat 112:381–388
Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R (1999) Experimental demonstration that offspring sex ratio varies with maternal condition. Proc Natl Acad Sci USA 96:570–573
Nilsson JA, Svensson E (1993) Energy constraints and ultimate decisions during egg-laying in the blue tit. Ecology 74:244–251
Oddie KR (1998) Sex discrimination before birth. Trends Ecol Evol 13:130–131
Oddie KR (2000) Size matters: competition between male and female great tit offspring. J Anim Ecol 69:903–912
Packard GC, Boardman TJ (1987) The misuse of ratios to scale physiological data that vary allometrically with body size. In: Feder EF, Bennett AF, Burggren WW, Huey RB (eds) New directions in ecological physiology. Cambridge University Press, Cambridge, pp 216–239
Parsons J (1970) Relationship between egg size and post-hatching chick mortality in the herring gull (Larus argentatus). Nature 228:1221–1222
Petrie M, Schwabl H, Brande-Lavridsen N, Burke T (2001) Sex differences in avian yolk hormone levels. Nature 412:498
Rasbash J, Browne W, Goldstein H, Yang M, Plewis I, Healy M, Woodhouse G, Draper D, Langford I, Lewis T (2000) A user's guide to MlwiN, 2nd edn. Institute of Education, London
Reid WV, Boersma PD (1990) Parental quality and selection on egg-size in the Magellanic penguin. Evolution 44:1780–1787
Schwabl H (1993) Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci USA 90:11446–11450
Sheldon BC (1998) Recent studies of avian sex ratio. Heredity 80:397–402
Smith HG, Bruun M (1998) The effect of egg size and habitat on starling nestling growth and survival. Oecologia 115:59–63
Smith HG, Ohlsson T, Wettermark K-J (1995) Adaptive significance of egg size in the European starling: experimental tests. Ecology 76:1–7
Stamps JA (1990) When should avian parents differentially provision sons and daughters? Am Nat 135:671–685
Stoleson SH, Beissinger SR (1995) Hatching asynchrony and the onset of incubation in birds, revisited. In: Power DM (ed) Current ornithology, vol 12. Plenum Press, New York, pp 191–270
Styrsky JD, Eckerle KP and Thompson CF (1999) Fitness-related consequences of egg mass innestling house wrens. Proc R Soc Lond B 266:1253–1258
Styrsky JD, Dobbs RC, Thompson CF (2000) Food-supplementation does not override the effect of egg mass on fitness-related traits of nestling house wrens. J Anim Ecol 69:690–702
Teather KL, Weatherhead PJ (1988) Sex-specific energy requirements of great-tailed grackle, Quiscalus mexicanus, nestlings. J Anim Ecol 57:659–668
Torres R, Drummond H (1999) Variably male-biased sex ratio in a marine bird with females larger than males. Oecologia 118:16–22
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
Velando A (2002) Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the blue-footed booby. Behav Ecol 13:443–449
Wiebe KL, Bortolotti GR (1992) Facultative sex ratio manipulation in American kestrels. Behav Ecol Sociobiol 30:379–386
Wiebe KL, Bortolotti GR (1995) Food-dependent benefits of hatching asynchrony in American kestrels Falco sparverius. Behav Ecol Sociobiol 36:49–57
Williams TD (1994) Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness. Biol Rev 68:35–59
Acknowledgements
We are very grateful to all those land holders and managers throughout the Riverina who allowed us to work and stay on their properties. Particular thanks go to the Jones, McLean, Clarke and Rutledge families on whose properties most of this work was conducted. We are also grateful to Mathew Berg, Wouter van Dongen, Alison Kemp, Emile van Lieshout, Marjan van Meerloo, Cordellia Moore, Arnoud van Petersen, Justin Welbergen and Iain Woxvold for their assistance in the field, and Martijn van de Pol for his help with the re-sampling analysis. We also thank the staff at the NSW National Parks and Wildlife Service (Griffith) and the Rural Lands and Protection board (Hillston and Hay) for advice and assistance. Useful comments on the manuscript were made by Mark Elgar, Theresa Jones, Mathew Symonds and two anonymous reviewers. This research was conducted with the approval of the University of Melbourne Animal Experimentation Ethics Committee, and financially supported by a grant JK from the Australian Research Council (A19802459). LB received financial support from the Marco Polo Fund!
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Dickinson
Rights and permissions
About this article
Cite this article
Magrath, M.J.L., Brouwer, L. & Komdeur, J. Egg size and laying order in relation to offspring sex in the extreme sexually size dimorphic brown songlark, Cinclorhamphus cruralis . Behav Ecol Sociobiol 54, 240–248 (2003). https://doi.org/10.1007/s00265-003-0627-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0627-y