Abstract
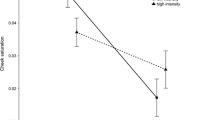
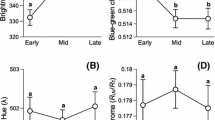
Many passerines lay protoporphyrin-pigmented eggs, and the degree of spotting seems to be related to female condition and environmental characteristics. However, most studies have ignored the relationship between the male’s quality and eggshell pigmentation. Because ornaments can act as honest indicators of individual quality, spottiness could be related to the parents’ feather colouration. Using models of bird vision, we investigated whether male and female ornamentation explained variation in spotting coverage in a free-living population of blue tits (Cyanistes caeruleus). We also explored the associations between other important individual characteristics (i.e. the pair’s infection status) and spotting coverage. Females that laid more pigmented eggs suffered from higher parasitaemia by the blood parasite Leucocytozoon and had smaller clutches, more saturated yellow breast feathers and reduced body mass. Male plumage colour and infection status explained a higher percentage of the variation in eggshell pigmentation than female characteristics. Males that had more saturated white cheeks and less saturated yellow breasts and were more intensely infected by the parasite Haemoproteus and less by Plasmodium attended nests with more spotted eggs. Additionally, these males were younger and more likely to father extra-pair offspring. These results, although observational, suggest that male attractiveness, male age, extra-pair paternity and parasitic infections could be important determinants of eggshell pigmentation. Males in poorer condition might have provided less food to laying females, which in turn laid more pigmented eggs and were also in poor condition. Alternatively, increased eggshell pigmentation could result from female differential allocation or breeding in low-quality territories.
Significance statement
Birds exhibit diverse egg colours and patterns, and these may vary in response to environmental characteristics, such as calcium availability, or female condition. The male’s characteristics may also partially determine egg appearance, although these remain understudied. In the blue tit, our results suggest that females laid more pigmented eggs when they were paired with males that cheated, were paler in their feather colour, and were more infected by parasites. These females were also in poor condition. Several hypotheses are proposed to explain the associations between male quality and the degree of spotting coverage on the eggshell. Because these males were in poorer condition, females could have been given less food and thus laid worse eggs or low-quality pairs could be breeding in low-quality territories. Alternatively, females could allocate more pigments to the eggshell depending on the male’s quality.


Similar content being viewed by others
References
Afonso S, Vanore G, Batlle A (1999) Protoporphyrin IX and oxidative stress. Free Radic Res 31:161–170
Amininasab S, Vedder O, Schut E, de Jong B, Magrath MJL, Korsten P, Komdeur J (2016) Influence of fine-scale habitat structure on nest-site occupancy, laying date and clutch size in Blue Tits Cyanistes caeruleus. Acta Oecol 70:37–44
Arct A, Drobniak SM, Cichoń M (2015) Genetic similarity between mates predicts extrapair paternity—a meta-analysis of bird studies. Behav Ecol 26:959–968
Avilés JM, Pérez-Contreras T, Navarro C, Soler JJ (2009) Male spotless starlings adjust feeding effort based on egg spots revealing ectoparasite load. Anim Behav 78:993–999
Badás EP, Martínez J, Rivero-de Aguilar J, Miranda F, Figuerola J, Merino S (2015) Ageing and reproduction: antioxidant supplementation alleviates telomere loss in wild birds. J Evol Biol 28:896–905
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188
Carrascal LM, Galván I, Gordo O (2009) Partial least squares regression as an alternative to current regression methods used in ecology. Oikos 118:681–690
Cassey P (2009) Biological optics: seeing colours in the dark. Curr Biol 19:1083–1084
Charmantier A, Blondel J, Perret P, Lambrechts MM (2004) Do extra-pair paternities provide genetic benefits for female blue tits Parus caeruleus? J Avian Biol 35:524–532
Cherry MI, Gosler AG (2010) Avian eggshell coloration: new perspectives on adaptive explanations. Biol J Linn Soc 100:753–762
Cohen J (1998) Statistical power analysis for the behavioral sciences, 2nd edn. Psychology Press, New York
Cuthill ICC (2006) Color perception. In: Hill GE, McGraw KJ (eds) Bird coloration. I. Mechanisms and measurements. Harvard University Press, Cambridge, MA, pp 3–40
Dakin R, Lendvai ÁZ, Ouyang JQ et al (2016) Plumage colour is associated with partner parental care in mutually ornamented tree swallows. Anim Behav 111:111–118
De Coster G, De Neve L, Lens L (2012) Intraclutch variation in avian eggshell pigmentation: the anaemia hypothesis. Oecologia 170:297–304
De Coster G, De Neve L, Lens L (2013) Intra-clutch variation in avian eggshell pigmentation covaries with female quality. J Ornithol 154:1057–1065
del Cerro S, Merino S, Martínez-de la Puente J et al (2010) Carotenoid-based plumage colouration is associated with blood parasite richness and stress protein levels in blue tits (Cyanistes caeruleus). Oecologia 162:825–835
Delhey K, Peters A, Johnsen A, Kempenaers B (2007) Fertilization success and UV ornamentation in blue tits Cyanistes caeruleus: correlational and experimental evidence. Behav Ecol 18:399–409
Doutrelant C, Gregoire A, Grnac N, Gomez D, Lambrechts MM, Perret P (2008) Female coloration indicates female reproductive capacity in blue tits. J Evol Biol 21:226–233
Duval C, Cassey P, Lovell PG, Mikšík I, Reynolds SJ, Spencer KA (2013a) Eggshell appearance does not signal maternal corticosterone exposure in Japanese quail: an experimental study with brown-spotted eggs. PLoS One 8:e80485
Duval C, Cassey P, Mikšík I, Reynolds SJ, Spencer KA (2013b) Condition-dependent strategies of eggshell pigmentation: an experimental study of Japanese quail (Coturnix coturnix japonica). J Exp Biol 216:700–708
Endler JA, Mielke PW (2005) Comparing entire color patterns as birds see them. Biol J Linn Soc 86:405–431
English PA, Montgomerie R (2011) Robin’s egg blue: does egg color influence male parental care? Behav Ecol Sociobiol 65:1029–1036
Evans SR, Hinks AE, Wilkin TA, Sheldon BC (2010) Age, sex and beauty: methodological dependence of age- and sex-dichromatism in the great tit Parus major. Biol J Linn Soc 101:777–796
Ferns P, Hinsley SA (2004) Immaculate tits: head plumage pattern as an indicator of quality in birds. Anim Behav 67:261–272
Flanagan GL, Morris S (1975) Window into a nest. Kestrel Books, Westerham, UK
Forstmeier W, Nakagawa S, Griffith SC, Kempenaers B (2014) Female extra-pair mating: adaptation or genetic constraint? Trends Ecol Evol 29:456–464
Galván I, Bonisoli-Alquati A, Jenkinson S, Ghanem G, Wakamatsu K, Mousseau TA, Møller AP (2014) Chronic exposure to low-dose radiation at Chernobyl favours adaptation to oxidative stress in birds. Funct Ecol 28:1387–1403
Galván I, Sanz JJ (2011) Mate-feeding has evolved as a compensatory energetic strategy that affects breeding success in birds. Behav Ecol 22:1088–1095
Garcia-Navas V, Ferrer ES, Bueno-Enciso J, Barrientos R, Sanz JJ, Ortego J (2014) Extrapair paternity in Mediterranean blue tits: socioecological factors and the opportunity for sexual selection. Behav Ecol 25:228–238
García-Navas V, Ferrer ES, Sanz JJ (2012) Plumage yellowness predicts foraging ability in the blue tit Cyanistes caeruleus. Biol J Linn Soc 106:418–429
García-Navas V, Sanz JJ, Merino S, Martínez-de la Puente J, Lobato E, del Cerro S, Rivero-de Aguilar J, Ruiz-De-Castañeda R, Moreno J (2011) Experimental evidence for the role of calcium in eggshell pigmentation pattern and breeding performance in Blue Tits Cyanistes caeruleus. J Ornithol 152:71–82
Giordano M, Costantini D, Pick JL, Tschirren B (2015) Female oxidative status, egg antioxidant protection and eggshell pigmentation: a supplemental feeding experiment in great tits. Behav Ecol Sociobiol 69:777–785
Giraudeau M, Duval C, Czirják GÁA, Bretagnole V, Eraud C, McGraw KJ, Heeb P (2011) Maternal investment of female mallards is influenced by male carotenoid-based coloration. Proc R Soc Lond B 278:781–788
Gomez D, Gregoire A, Del Rey GM, Bassoul M, Degueldre D, Perret P, Doutrelant C (2014) The intensity threshold of colour vision in a passerine bird, the blue tit. J Exp Biol 217:3775–3778
Gorchein A, Lim C, Cassey P (2009) Extraction and analysis of colourful eggshell pigments using HPLC and HPLC/electrospray ionization tandem mass spectrometry. Biomed Chromatogr 23:602–606
Gosler AG, Barnett PR, Reynolds SJ (2000) Inheritance and variation in eggshell patterning in the great tit Parus major. Proc R Soc Lond B 270:2469–2473
Gosler AG, Higham JP, Reynolds SJ (2005) Why are birds’ eggs speckled? Ecol Lett 8:1105–1113
Griggio M, Serra L, Licheri D, Campomori C, Pilastro A (2009) Moult speed affects structural feather ornaments in the blue tit. J Evol Biol 22:782–792
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Hanssen SA, Folstad I, Erikstad KE (2006) White plumage reflects individual quality in female eiders. Anim Behav 71:337–343
Hargitai R, Herényi M, Török J (2008) Eggshell coloration in relation to male ornamentation, female condition and egg quality in the collared flycatcher Ficedula albicollis. J Avian Biol 39:413–422
Hargitai R, Nagy G, Herényi M, Nyiri Z, Laczi M, Hegyi G, Eke Z, Török J (2016) Darker eggshell spotting indicates lower yolk antioxidant level and poorer female quality in the Eurasian Great Tit (Parus major). Auk 133:131–146
Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S (2011) Carry-over effects as drivers of fitness differences in animals. J Anim Ecol 80:4–18
Hart NS, Partridge JC, Cuthill IC, Bennett ATD (2000) Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.) J Comp Physiol A 186:375–387
Hill GE (2006a) Female mate choice for ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration. II. Function and evolution. Harvard University Press, Cambridge, MA, pp 137–200
Hill GE (2006b) Environmental regulation of ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration. I. Mechanisms and measurements. Harvard University Press, Cambridge, MA, pp 507–560
Hill GE, Farmer KL, Beck ML (2004) The effect of mycoplasmosis on carotenoid plumage coloration in male house finches. J Exp Biol 207:2095–2099
Holveck MJ, Doutrelant C, Guerreiro R, Perret P, Gomez D, Grégoire A (2010) Can eggs in a cavity be a female secondary sexual signal? Male nest visits and modelling of egg visual discrimination in blue tits. Biol Lett 6:453–457
Holveck MJ, Grégoire A, Staszewski V, Staszewski V, Guerreiro R, Perret P, Boulinier T, Doutrelant C (2012) Eggshell spottiness reflects maternally transferred antibodies in blue tits. PLoS One 7:e50389
Jakobowicz E, Derquenne C (2007) A modified PLS path modeling algorithm handling reflective categorical variables and a new model building strategy. Comput Stat Data An 51:3666–3678
Jones CD, Osorio D (2004) Discrimination of oriented visual textures by poultry chicks. Vis Res 44:83–89
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Kemp DJ, Herberstein ME, Fleishman LJ, Endler JA, Bennett AT, Dyer AG, Hart NS, Marshall J, Whiting MJ (2015) An integrative framework for the appraisal of coloration in nature. Am Nat 185:705–724
Kempenaers B, Schlicht E (2010) Extra-pair behaviour. In: Kappeler P (ed) Animal behavior: evolution and mechanisms. Springer, Heidelberg, pp 359–411
Kempenaers B, Verheyen GR, Dhondt AA (1995) Mate guarding and copulation behaviour in monogamous and polygynous blue tits: do males follow a best-of-a-bad-job strategy? Behav Ecol Sociobiol 36:33–42
Komdeur J, Oorebeek M, van Overveld T, Cuthill I (2005) Mutual ornamentation, age, and reproductive performance in the European starling. Behav Ecol 16:805–817
Lachish S, Knowles SCL, Alves R, Wood MJ, Sheldon B (2011) Fitness effects of endemic malaria infections in a wild bird population: the importance of ecological structure. J Anim Ecol 80:1196–1206
Lahti D, Ardia D (2016) Shedding light on bird egg color: pigment as parasol and the dark car effect. Am Nat 187:547–563
López-Rull I, Celis P, Gil D (2007) Egg colour covaries with female expression of a male ornament in the spotless starling (Sturnus unicolor). Ethology 113:926–933
Martínez-de la Puente J, Merino S, Moreno J, Tomás G, Morales J, Lobato E, García-Fraile S, Martínez J (2007) Are eggshell spottiness and colour indicators of health and condition in blue tits Cyanistes caeruleus? J Avian Biol 38:377–384
Martínez-de la Puente J, Merino S, Tomás G, Moreno J, Morales J, Lobato E, García-Fraile S, Belda EJ (2010) The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol Lett 6:663–665
Martínez-Padilla J, Dixon H, Vergara P, Pérez-Rodríguez L, Fargallo JA (2010) Does egg colouration reflect male condition in birds? Naturwissenschaften 97:469–477
Marzal A, De Lope F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545
Maurer G, Portugal SJ, Cassey P (2011) Review: an embryo’s eye view of avian eggshell pigmentation. J Avian Biol 42:494–504
McGraw KJ (2006a) Mechanics of uncommon colors: pterins, porphyrins, and psittacofulvins. In: Hill GE, McGraw KJ (eds) Bird coloration. I. Mechanisms and measurements. Harvard University Press, Cambridge, MA, pp 354–398
McGraw KJ (2006b) The mechanics of carotenoid coloration in birds. In: Hill GE, McGraw KJ (eds) Bird coloration. I. Mechanisms and measurements. Harvard University Press, Cambridge, MA, pp 177–242
McGraw KJ, Hill GE, Parker R (2005) The physiological costs of being colourful: nutritional control of carotenoid utilization in the American goldfinch, Carduelis tristis. Anim Behav 69:653–660
Merino S, Moreno J, Sanz JJ, Arriero E (2000) Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc R Soc Lond B 267:2507–2510
Merino S, Potti J, Fargallo JA (1997) Blood parasites of passerine birds from central Spain. J Wildlife Dis 33:638–641
Møller AP, Szép T (2002) Survival rate of adult barn swallows Hirundo rustica in relation to sexual selection and reproduction. Ecology 83:2220–2228
Moreno J, Osorno JL (2003) Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806
Moreno J, Velando A, Ruiz-De-Castañeda R, Cantarero A, González-Braojos S, Redondo A (2011) Plasma antioxidant capacity and oxidative damage in relation to male plumage ornamental traits in a montane Iberian pied flycatcher Ficedula hypoleuca population. Acta Ornithol 46:65–70
O’Brien EL, Dawson RD (2011) Plumage color and food availability affect male reproductive success in a socially monogamous bird. Behav Ecol 22:66–72
Osorio D, Vorobyev M, Jones C (1999) Colour vision of domestic chicks. J Exp Biol 202:2951–2959
Otter KA, Atherton SE, van Oort H (2007) Female food solicitation calling, hunger levels and habitat differences in the black-capped chickadee. Anim Behav 74:847–853
Perrins CM (1970) The timing of birds’ breeding seasons. Ibis 112:242–255
Prum RO (2006) Anatomy, physics and evolution of structural colors. In: Hill GE, McGraw KJ (eds) Bird coloration. I. Mechanisms and measurements. Harvard University Press, Cambridge, MA, pp 295–353
R Development Core Team (2015) R: a language and environment for statistical computing, version 3.1.3. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Reynolds SJ, Martin GR, Cassey P (2009) Is sexual selection blurring the functional significance of eggshell coloration hypotheses? Anim Behav 78:209–215
Robb GN, McDonald RA, Chamberlain DE, Reynolds SJ, Harrison TJ, Bearhop S (2008) Winter feeding of birds increases productivity in the subsequent breeding season. Biol Lett 4:220–223
Royama T (1966) A re-interpretation of courtship feeding. Bird Study 13:116–129
Sanz JJ, García-Navas V (2009) Eggshell pigmentation pattern in relation to breeding performance of blue tits Cyanistes caeruleus. J Anim Ecol 78:31–41
Senar JC (2002) Great tits (Parus major) reduce body mass in response to wing area reduction: a field experiment. Behav Ecol 13:725–727
Sheldon BC (2000) Differential allocation: tests, mechanisms and implications. Trends Ecol Evol 15:397–402
Sheldon BC, Andersson S, Griffith SC, Örnborg J, Sendecka J (1999) Ultraviolet colour variation influences blue tit sex ratios. Nature 402:874–877
Soler JJ, Navarro C, Contreras TP, Avilés JM, Cuervo JJ (2008) Sexually selected egg coloration in spotless starlings. Am Nat 171:183–194
Spottiswoode CN, Stevens M (2011) How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc R Soc Lond B 278:3566–3573
Stevens M (2011) Avian vision and egg colouration: concepts and measurements. Avian Biol Res 4:168–184
Stevens M, Lown AE, Wood LE (2014) Color change and camouflage in juvenile shore crabs Carcinus maenas. Front Ecol Evol 2:14
Stevens M, Stoddard M, Higham J (2009) Studying primate color: towards visual system-dependent methods. Int J Primatol 30:893–917
Stoddard MC, Prum RO (2008) Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of new world buntings. Am Nat 171:755–776
Svensson L (1992) Identification guide to European passerines. Natural History Museum, Stockholm
Trigo S, Mota PG (2016) Carotenoid-based plumage colouration is predicted by age and parasites in the male European serin. J Avian Biol 47:409–416
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine Press, Chicago, IL, pp 136–179
Valkiūnas G (2005) Avian malaria parasites and other Haemosporidia. CRC Press, Boca Raton, FL, USA
Vedder O, Komdeur J, van der Velde M, Schut E, Magrath MJ (2011) Polygyny and extra-pair paternity enhance the opportunity for sexual selection in blue tits. Behav Ecol Sociobiol 65:741–752
Velando A, Beamonte-Barrientos R, Torres R (2006) Pigment-based skin colour in the blue-footed booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149:535–542
Vorobyev M, Osorio D, Bennett AT, Marshall NJ, Cuthill IC (1998) Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol A 183:621–633
Walker LK, Stevens M, Karadaş F, Kilner RM, Ewen JG (2013) A window on the past: male ornamental plumage reveals the quality of their early-life environment. Proc R Soc B 280:20122852
Wang XT, Zhao CJ, Li JY, Xu GY, Lian LS, Wu CX, Deng XM (2009) Comparison of the total amount of eggshell pigments in Dongxiang brown-shelled eggs and Dongxiang blue-shelled eggs. Poultry Sci 88:1735–1739
Wegmann M, Vallat-Michel A, Richner H (2015) An evaluation of different methods for assessing eggshell pigmentation and pigment concentration using great tit eggs. J Avian Biol 46:597–607
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab 58:109–130
Yuta T, Koizumi I (2016) Does nest predation risk affect the frequency of extra-pair paternity in a socially monogamous passerinse? J Avian Biol 47:153–158
Acknowledgments
We thank ‘El Ventorrillo’ field station for use of their facilities and field assistants that offered help during fieldwork. We are indebted to L. M. Carrascal for his comments on the statistical analyses, L.M. Arenas and G. McIvor, and two anonymous reviewers for valuable comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by projects CGL2012-40026-C02-01 to SM and CGL2012-40026-C02-02 to JM from the MEC (Ministerio de Economia y Competitividad). EPB was supported by a JAE grant from the Spanish National Research Council (CSIC).
Conflict of interest
EPB has received a research JAE grant from the Spanish National Research Council (CSIC). The other authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and national guidelines for the care and use of animals were followed. Researchers manipulating birds held a specific ringing permit with permission from J. Donés (Director of ‘Montes de Valsain’).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by K. McGraw
Electronic supplementary material
ESM 1
(DOCX 778 kb)
Rights and permissions
About this article
Cite this article
Badás, E.P., Martínez, J., Rivero-de Aguilar, J. et al. Eggshell pigmentation in the blue tit: male quality matters. Behav Ecol Sociobiol 71, 57 (2017). https://doi.org/10.1007/s00265-017-2286-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2286-4