Abstract
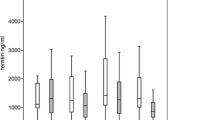
The majority of patients with myelodysplastic syndrome (MDS) present with anemia and will become dependent on regular transfusions of packed red blood cells (PRBC) with the risk of iron overload (IOL). Liver iron content best reflects the total body iron content, and measurement of liver iron concentration (LIC) by MRI is a validated tool for detection, but data in MDS is rather limited. Here we present the results of a multi-center trial evaluating the efficacy and safety of deferasirox (DFX) in low and intermediate-1 risk MDS patients with transfusion-dependent IOL. Three patients with transfusion frequency of > 4 units PRBC per month were initially treated with 30 mg/kg/day while in 46 patients with a lower transfusion burden deferasirox was initiated at 20 mg/kg/day, due to patient related reasons one patient received DFX in a dose of 6 mg/kg/day only. LIC was measured by MRI at baseline and end of study using the method by St. Pierre et al. The intention to treat population consisted of 50 MDS patients (28 male; 22 female) with a median age of 69 years who were treated with DFX for a median duration of 354 days. Mean daily dose of DFX was 19 mg/kg/day. Median serum ferritin level (SF) at baseline was 2,447 ng/mL and decreased to 1,685 ng/mL (reduction by 31 %) at end of study (p = 0.01). In 7 (13 %) patients the initially chosen dose had to be increased due to unsatisfactory efficacy of chelation therapy. For 21 patients, LIC measurement by liver MRI was performed at baseline and for 19 of these patients at the end of study: mean LIC decreased significantly from 16,8 mg/g dry tissue weight (± 8.3 mg/g dry tissue weight) at study entry to 10,8 mg/g dry tissue weight (± 10.4 mg/g dry tissue weight) at end of study (p = 0.01). Of all patients exposed to the study drug (n = 54), 28 (52 %) did not complete the 12 month study period most commonly due to AEs in 28 % (n = 15) and abnormal laboratory values in 7 % (n = 4), respectively. The most common adverse events (≥ 10 % of all patients) with suspected drug relationship were diarrhea (n = 25, 46 %), nausea (n = 13, 24 %), upper abdominal pain (n = 8, 15 %), serum creatinine increase (n = 16, 30 %) and rash (n = 5, 9 %). Adverse events making dose adjustments or interruption of study drug necessary occurred in 33 patients (61 %). Hematologic improvement according to IWG criteria (2006) was observed in 6 patients (11 %). Initiation of treatment of IOL with DFX depending on the transfusion burden yields sufficient reduction of excess iron indicated by serum ferritin levels and most importantly by liver MRI. The safety profile of DFX was comparable to previous observations.


Similar content being viewed by others
References
Nolte F, Hofmann WK (2010) Molecular mechanisms involved in the progression of myelodysplastic syndrome. Future Oncol 6:445–455
Kao JM, McMillan A, Greenberg PL (2008) International MDS risk analysis workshop (IMRAW)/IPSS reanalyzed: impact of cytopenias on clinical outcomes in myelodysplastic syndromes. Am J Hematol 83:765–770
Neukirchen J, Schoonen WM, Strupp C et al (2011) Incidence and prevalence of myelodysplastic syndromes: data from the Dusseldorf MDS-registry. Leuk Res 35:1591–1596
Gattermann N, Rachmilewitz EA (2011) Iron overload in MDS-pathophysiology, diagnosis, and complications. Ann Hematol 90:1–10
Modell B, Khan M, Darlison M (2000) Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 355:2051–2052
Borgna-Pignatti C, Rugolotto S, De Stefano P et al (2004) Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 89:1187–1193
Takatoku M, Uchiyama T, Okamoto S et al (2007) Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol 78:487–494
Leitch HA (2007) Improving clinical outcome in patients with myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk Res 31(Suppl 3):S7–S9
Rose C, Brechignac S, Vassilief D et al (2010) Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myelodysplasies). Leuk Res 34:864–870
Steensma DP (2011) The role of iron chelation therapy for patients with myelodysplastic syndromes. J Natl Compr Canc Netw 9:65–75
Arboretti R, Tognoni G, Alberti D (2001) Pharmacosurveillance and quality of care of thalassaemic patients. A large scale epidemiological survey. Eur J Clin Pharmacol 56:915–922
Jensen PD, Jensen IM, Ellegaard J (1992) Desferrioxamine treatment reduces blood transfusion requirements in patients with myelodysplastic syndrome. Br J Haematol 80:121–124
Jensen PD, Heickendorff L, Pedersen B et al (1996) The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol 94:288–299
Guariglia R, Martorelli MC, Villani O et al (2011) Positive effects on hematopoiesis in patients with myelodysplastic syndrome receiving deferasirox as oral iron chelation therapy: a brief review. Leuk Res 35:566–570
Gattermann N, Finelli C, Della Porta M et al (2012) Hematologic responses with deferasirox therapy in transfusion-dependent myelodysplastic syndromes patients. Haematologica 97:1364–1371
Breccia M, Finsinger P, Loglisci G et al (2012) Deferasirox treatment for myelodysplastic syndromes: “real-life” efficacy and safety in a single-institution patient population. Ann Hematol 91:1345–1349
List AF, Baer MR, Steensma DP et al (2012) Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J Clin Oncol 30:2134–2139
Hartmann J, Sinzig U, Wulf G et al (2009) Significant suppression of the colony forming capacity of erythroid progenitors by iron overload in patients with MDS and sequential analyses of BFU-E under chelation therapy. ASH Annu Meet Abstr 114:3826
Greenberg PL, Koller CA, Cabantchik ZI et al (2010) Prospective assessment of effects on iron-overload parameters of deferasirox therapy in patients with myelodysplastic syndromes. Leuk Res 34:1560–1565
Cappellini MD, Cohen A, Piga A et al (2006) A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood 107:3455–3462
Piga A, Galanello R, Forni GL et al (2006) Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica 91:873–880
St Pierre TG, Clark PR, Chua-anusorn W et al (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105:855–861
Gattermann N, Finelli C, Porta MD et al (2010) Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: results from the large 1-year EPIC study. Leuk Res 34:1143–1150
List AF, Baer MR, Steensma D et al (2008) Iron chelation with deferasirox (Exjade(R)) improves iron burden in patients with myelodysplastic syndromes (MDS). ASH Annu Meet Abstr 112:634
Lee JW, Yoon SS, Shen ZX et al (2010) Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood 116:2448–2454
Bennett JM (2008) Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol 83:858–861
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nolte, F., Höchsmann, B., Giagounidis, A. et al. Results from a 1-year, open-label, single arm, multi-center trial evaluating the efficacy and safety of oral Deferasirox in patients diagnosed with low and int-1 risk myelodysplastic syndrome (MDS) and transfusion-dependent iron overload. Ann Hematol 92, 191–198 (2013). https://doi.org/10.1007/s00277-012-1594-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-012-1594-z