Abstract
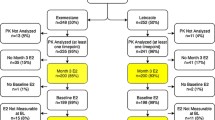
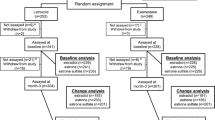
Objective:A randomized multicenter (14 centers) trial was conducted in 114 men with prostate cancer to determine whether the antiestrogen tamoxifen (‘Nolvadex’) 20 mg or the aromatase inhibitor anastrozole (‘Arimidex’) 1 mg prevent gynecomastia and breast pain during treatment with the non-steroidal antiandrogen bicalutamide (‘Casodex’) 150 mg, without compromising efficacy, safety, or quality of life. Plasma samples were collected in a subgroup of these patients to investigate whether trough (pre-dose) concentrations of bicalutamide 150 mg are influenced by concomitant administration of tamoxifen 20 mg or anastrozole 1 mg; the results of this pilot study are reported in this article. Methods: A subpopulation of patients from a randomized placebo-controlled trial evaluating tamoxifen 20 mg and anastrozole 1 mg for the prevention of gynecomastia and breast pain in men receiving bicalutamide 150 mg for early or recurrent prostate cancer were selected on a voluntary basis from three of the trial centers. Plasma samples were collected on days 7, 14, 28, and 84 of therapy and analyzed to determine the plasma concentrations of (R)–bicalutamide and (S)-bicalutamide. In addition, plasma concentrations of tamoxifen, N–desmethyltamoxifen, and anastrozole were determined. Results: A total of 21 patients were selected. There was no significant difference between treatment groups with respect to the trough plasma concentrations of either bicalutamide enantiomer at any point during the study. Plasma concentrations of the enantiomers, and the relative proportion of the ®)- and (S)–enantiomers, were consistent with those reported in previous studies. Plasma concentrations of tamoxifen, N–desmethyltamoxifen, and anastrozole were also similar to those described elsewhere in the literature. Conclusions: The findings of this pilot study suggest that trough plasma concentrations of bicalutamide enantiomers following administration of bicalutamide 150 mg are not markedly influenced by concomitant administration of tamoxifen 20 mg or anastrozole 1 mg. However, an effect of tamoxifen on bicalutamide pharmacokinetics can not be completely excluded due to the size of this study. Further studies are needed to clarify the effect of tamoxifen on bicalutamide pharmacokinetics and prostate cancer control in bicalutamide-treated patients. ‘Arimidex’, ‘Casodex’, and ‘Nolvadex’ are trademarks of the AstraZeneca group of companies
Similar content being viewed by others
References
Boccardo F, Rubagotti A, Garofalo L, Di Tonno P, Conti G, Bertaccini A, De Antoni P, Galassi P, Siragusa A, Durand F (2003) Tamoxifen is more effective than anastrozole in preventing gynecomastia and breast pain induced by bicalutamide monotherapy in prostate cancer patients. Proc Am Soc Clin Oncol 22:400 (abstract)
Buzdar AU (2003) Pharmacology and pharmacokinetics of the newer generation aromatase inhibitors. Clin Cancer Res 9:468S–472S
Cockshott ID (2004) Bicalutamide: clinical pharmacokinetics and metabolism. Clin Pharmacokinet (in press)
Kaisary A, Klarskov P, McKillop D (1996) Absence of hepatic enzyme induction in prostate cancer patients receiving ‘Casodex’ (bicalutamide). Anticancer Drugs 7:54–59
McLeod DG, Iversen P (2000) Gynecomastia in patients with prostate cancer: a review of treatment options. Urology 56:713–720
Morello KC, Wurz GT, DeGregorio MW (2003) Pharmacokinetics of selective estrogen receptor modulators. Clin Pharmacokinet 42:361–372
Saltzstein D, Cantwell A, Sieber P, Ross JR, Silvay-Mandeau O, Gallo J (2002) Prophylactic tamoxifen significantly reduces the incidence of bicalutamide-induced gynaecomastia and breast pain. BJU Int 90 (Suppl 2):120–121
See WA, McLeod D, Iversen P, Wirth M (2001) The bicalutamide Early Prostate Cancer program: demography. Urol Oncol 6:43–47
See WA, Wirth MP, McLeod DG, Iversen P, Klimberg I, Gleason D, Chodak G, Montie J, Tyrrell C, Wallace DMA, Delaere KPJ, Vaage S, Tammela TLJ, Lukkarinen O, Persson B-E, Carroll K, Kolvenbag GJCM, on behalf of the Casodex Early Prostate Cancer Trialist Group (2002) Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: first analysis of the early prostate cancer program. J Urol 168:429–435
Soininen K, Kleimola T, Elomaa I, Salmo M, Rissanen P (1986) The steady-state pharmacokinetics of tamoxifen and its metabolites in breast cancer patients. J Int Med Res 14:162–165
Tyrrell CJ, Denis L, Newling D, Soloway M, Channer K, Cockshott ID, on behalf of the ‘Casodex’ Study Group (1998) CasodexTM 10–200 mg daily, used as monotherapy for the treatment of patients with advanced prostate cancer. An overview of the efficacy, tolerability and pharmacokinetics from three Phase II dose-ranging studies. Eur Urol 33:39–53
Wiebe VJ, Benz CC, DeGregorio MW (1988) Clinical pharmacokinetics of drugs used in the treatment of breast cancer. Clin Pharmacokinet 15:180–193
Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Carroll K, on behalf of the ‘Casodex’ Early Prostate Cancer Trialists’ Group (2004) Bicalutamide (‘Casodex’) 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at 5.4 years’ median follow-up. J Urol (in press)
Acknowledgements
The authors gratefully acknowledge the assistance of BAS Analytics (Kenilworth, Warwickshire, UK) and MDS Pharma Services (Blainville, QC, Canada) in determining the drug plasma levels reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
This trial was sponsored by AstraZeneca (Milan, Italy)
Rights and permissions
About this article
Cite this article
Boccardo, F., Rubagotti, A., Conti, G. et al. Exploratory study of drug plasma levels during bicalutamide 150 mg therapy co-administered with tamoxifen or anastrozole for prophylaxis of gynecomastia and breast pain in men with prostate cancer. Cancer Chemother Pharmacol 56, 415–420 (2005). https://doi.org/10.1007/s00280-005-1016-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-1016-1