Abstract
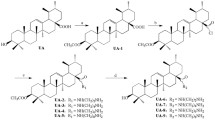
Purpose: Since 2-deoxy-D-glucose (2-DG) is currently in phase I clinical trials to selectively target slow-growing hypoxic tumor cells, 2-halogenated D-glucose analogs were synthesized for improved activity. Given the fact that 2-DG competes with D-glucose for binding to hexokinase, in silico modeling of molecular interactions between hexokinase I and these new analogs was used to determine whether binding energies correlate with biological effects, i.e. inhibition of glycolysis and subsequent toxicity in hypoxic tumor cells. Methods and Results: Using a QSAR-like approach along with a flexible docking strategy, it was determined that the binding affinities of the analogs to hexokinase I decrease as a function of increasing halogen size as follows: 2-fluoro-2-deoxy-D-glucose (2-FG) > 2-chloro-2-deoxy-D-glucose (2-CG) > 2-bromo-2-deoxy-D-glucose (2-BG). Furthermore, D-glucose was found to have the highest affinity followed by 2-FG and 2-DG, respectively. Similarly, flow cytometry and trypan blue exclusion assays showed that the efficacy of the halogenated analogs in preferentially inhibiting growth and killing hypoxic vs. aerobic cells increases as a function of their relative binding affinities. These results correlate with the inhibition of glycolysis as measured by lactate inhibition, i.e. ID50 1 mM for 2-FG, 6 mM for 2-CG and > 6 mM for 2-BG. Moreover, 2-FG was found to be more potent than 2-DG for both glycolytic inhibition and cytotoxicity. Conclusions: Overall, our in vitro results suggest that 2-FG is more potent than 2-DG in killing hypoxic tumor cells, and therefore may be more clinically effective when combined with standard chemotherapeutic protocols.





Similar content being viewed by others
References
Adamson J, Foster AB (1969) 2-Chloro-2-deoxy-D-glucose and 2,2-dichloro-2-deoxy-D-arabino-hexose. Carb Res 10:517–523
Albert M, Dax K, Ortner J (1998) A novel direct route to 2-deoxy-2-fluoro-aldoses and their corresponding derivatives. Tetrahedron 54:4839–4848
Aleshin AE, Kirby C, Liu XF et al (2000) Crystal structures of mutant monomeric hexokinase I reveal multiple ADP binding sites and conformational changes relevant to allosteric regulation. J Mol Biol 296:1001–1015
Bessell EM, Foster AB, Westwood JH (1972) The use of deoxyfluoro-D-glucopyranoses and related compounds in a study of yeast hexokinase specificity. Biochem J 128:199–204
Brown J (1962) Effects of 2-deoxyglucose on carbohydrate metabolism: review of the literature and studies in the rat. Metabolism 11:1098
Crane RK, Sols A (1954) The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem 210:597–606
Di Raddo P, Diksic M (1986) Fluorination of 3,4,6-tri-O-acetyl-1,5-anhydro-2-deoxy-D-arabino-hex-1-enitol in water. Carb Res 153:141–145
Gordon RK, Ginalski K, Rudnicki WR et al (2003) Anti-HIV-1 activity of 3-deaza-adenosine analogs—Inhibition of S-adenosylhomocysteine hydrolase and nucleotide congeners. Eur J Biochem 270:3507–3517
Greijer AE, van der Wall E (2004) The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol 57:1009–1014
Hoekstra C, Paglianiti I, Hoekstra O (2000) Monitoring response to therapy in cancer using [18F]-2-fluoro-2-deoxy-D-glucose and positron emission tomography: an overview of different analytical methods. Eur J Nucl Med 27:731–743
Hwang M-J, Stockfisch TP, Hagler AT (1994) Derivation of class II force fields. II. Derivation and characterization of a class II force field, CFF93, for the alkyl functional group and alkane molecules. J Am Chem Soc 116:2515–2525
Kalir T, Wang BY, Goldfischer M, Haber RS et al (2002) Immunohistochemical staining of GLUT1 in benign, borderline, and malignant ovarian epithelia. Cancer 94:1078–1082
Kamata K, Mitsuya M, Nishimura T et al (2004) Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure 12:429–438
King MP, Attardi G (1989) Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246:500–503
Krishan A, Paika K, Frei E (1975) Cytofluorometric studies on the action of podophyllotoxin and epipodophyllotoxins (VM-26, VP-16-213) on the cell cycle traverse of human lymphoblasts. J Cell Biol 66:52
Lampidis TJ, Priebe W (2003) Cancer chemotherapy with 2-deoxy-D-glucose. US Patent 6,670,330,B1, 30 Dec 2003
Larson S, Grunbaum Z, Rasey J (1980) Positron imaging feasibility studies: selective tumor concentration of 3H-thymidine, 3H-uridine, and 14C-2-deoxyglucose. Radiology 134:771–773
Liu H, Hu YP, Savaraj N, Priebe W, Lampidis TJ (2001) Hypersensitization of tumor cells to glycolytic inhibitors. Biochemistry 40:5542–5547
Liu X, Kim CS, Kurbanov FT et al (1999) Dual mechanisms for glucose 6-phosphate inhibition of human brain hexokinase. J Biol Chem 274:31155–1159
Liu H, Savaraj N, Priebe W, Lampidis TJ (2002) Hypoxia increases tumor cell sensitivity to glycolytic inhibitors a strategy for solid tumor therapy (Model C). Biochem Pharmacol 64:1746–1751
Maher JC, Krishan A, Lampidis TJ (2004) Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic versus aerobic conditions. Cancer Chemother Pharmacol 53:116–122
McKeehan WL (1982) Glycolysis, glutaminolysis, and cell proliferation. Cell Biol Int Rep 6:635–650
Maple JR, Hwang M-J, Stockfisch TP et al (1994) Derivation of class II force fields. I. Methodology and quantum force field for the alkyl functional group and alkane molecules. J Comput Chem 15:162–182
Maschek G, Savaraj N, Priebe W (2004) 2-Deoxy-D-Glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res 64:31–34
Mazurek S, Boschek CB, Eigenbrodt E (1997) The role of phosphometabolites in cell proliferation, energy metabolism, and tumor therapy. J Bioenerg Biomembr 29:315–327
Nirenberg MW, Hogg JF (1958) Inhibition of anaerobic glycolysis in Ehrlich ascites tumor cells by 2-deoxy-D-glucose. Cancer Res 18:518
Noguchi Y, Marat D, Saito A et al (1999) Expression of facilitative glucose transporters in gastric tumors. Hepatogastroenterology 46:2683–2689
Ortner J, Albert M, Weber H, Dax K (1999) Studies on the reaction of D-glucal and its derivatives with 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane salts. J Carb Chem 18:297–316
Rappe AK, Goddard WAI (1991) Charge equilibration for molecular dynamics simulations. J Phys Chem 95:3358–3363
Reitzer LJ, Wice BM, Kennell D (1979) Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem 254:2669–2676
Reitzer LJ, Wice BM, Kennell D (1980) The pentose cycle: control and essential function in HeLa cell nucleic acid synthesis. J Biol Chem 255:5616–5626
Rohren EM, Turkington TG, Coleman RE (2004) Clinical applications of PET in oncology. Radiology 231:305–332
Rudlowski C, Becker AJ, Schroder W et al (2003) GLUT1 messenger RNA and protein induction relates to the malignant transformation of cervical cancer. Am J Clin Pathol 120:691–698
Rudnicki WR, Kurzepa M, Szczepanik T et al (2000) A simple model for predicting the free energy of binding between anthracycline antibiotics and DNA. Acta Biochim Pol 47:1–9
Schmidt MF, Biely P, Kratky Z, Schwarz RT (1978) Metabolism of 2-deoxy-2-fluoro-D-[3H]glucose and 2-deoxy-2-fluoro-D-[3H]mannose in yeast and chick-embryo cells. Eur J Biochem 87:55–68
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 10:721–732
Sharma V, Luker GD, Piwnica-Worms D (2002) Molecular imaging of gene expression and protein function in vivo with PET and SPECT. J Magn Reson Imaging 16: 336–351
Wachsberger PR, Gressen EL, Bhala A et al (2002) Variability in glucose transporter-1 levels and hexokinase activity in human melanoma. Melanoma Res 12:35–43
Teichman M, Descotes G, Lafont D (1993) Bromination of 1,5-anhydrohex-1-enitols (glycals) using quarternary ammonium tribromides as bromine donors: synthesis of α-1,2-trans-2-bromo-2-deoxyglycopyranosyl bromides and fluorides. Synthesis 889–894
Wahl R, Hutchins G, Buchsbaum D (1991) 18F-2-deoxy-2-fluoro-D-glucose (FDG) uptake into human tumor xenografts: feasibility studies for cancer imaging with PET. Cancer 67:1544–1550
Wick AN, Drury DR, Nakada HI, Wolfe JB (1957) Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem 224:963
Zingone A, Seidel J, Aloj L et al (2002) Monitoring the correction of glycogen storage disease type 1a in a mouse model using [(18) F] FDG and a dedicated animal scanner. Life Sci 71:1293–1301
Acknowledgment
Supported by NIH grant R01 CA037109 16, SPORE grant in Pancreatic Cancer P20 CA101936, a grant from The Morton and Angela Topfer Pancreatic Cancer Research Fund, and BST funds of Warsaw University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lampidis, T.J., Kurtoglu, M., Maher, J.C. et al. Efficacy of 2-halogen substituted d-glucose analogs in blocking glycolysis and killing “hypoxic tumor cells”. Cancer Chemother Pharmacol 58, 725–734 (2006). https://doi.org/10.1007/s00280-006-0207-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0207-8