Abstract
Background
Docetaxel has marked inter-patient PK variability, and metabolic phenotypic probes may enable individualised dosing. This is the first report directly comparing the erythromycin breath test (EBT) (a CYP3A4 probe) with the antipyrine clearance test (ACT), (a general CYP-P450/predominant CYP3A4 probe) for the correlation with docetaxel PK and toxicity.
Methods
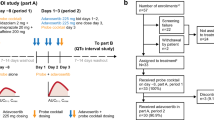
Patients pretherapy underwent: (A) EBT: IV C14[N-methyl]-erythromycin was administered and breath samples analysed for 14CO2, derived parameters included (1) 14CO2 flux at 10-min (CO2f10), (2) 20-min (CO2f20), (3) terminal rate constant kCO2 and (4) AUCCO2,(0–∞) and AUCCO2,(0–60). (B) ACL test: patients were given oral antipyrine 10 mg/kg, blood samples were taken for PK, and the clearance (CLAnt) was derived. Docetaxel was then given at 75 mg/m2/3-weekly or 35 mg/m2/weekly. Samples taken for docetaxel PK in first course on day 1 and PK parameters included clearance (CLDoc).
Results
Twenty patients accrued, docetaxel: 3-weekly/weekly = 13:7. EBT parameters (N = 19) (mean, [CV%]): CO2f10 (%/min) 0.051 (106), CO2f20 0.052 (82), kCO2 (min−1) 0.007 (22), AUCCO2,(0–∞) 7.9 (85), AUCCO2,(0–60) 2.64 (81). CLAnt (N = 19) (ml/min); 35.8 (37). Docetaxel PK parameters (N = 19): CLDoc (l/h) = 57.2 (36), tDoc1/2 (h) = 12.7 (33). No correlations were observed between the docetaxel PK and EBT parameters. For docetaxel weekly patients, a significant linear relationship was observed between CLDoc and CLAnt (P = 0.007, R 2 = 79.47%).
Conclusions
The utility of EBT for the prediction of docetaxel PK was not confirmed in this study. The antipyrine clearance test may be superior in this regard for docetaxel, but regimen dependent and hence warrants further evaluation.

Similar content being viewed by others
References
Baker SD, Verweij J, Cusatis GA, van Schaik RH, Marsh S, Orwick SJ, Franke RM, Hu S, Schuetz EG, Lamba V, Messersmith WA, Wolff AC, Carducci MA, Sparreboom A (2009) Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther 85:155–163
Baker SD, Zhao M, Lee CK, Verweij J, Zabelina Y, Brahmer JR, Wolff AC, Sparreboom A, Carducci MA (2004) Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res 10:1976–1983
Baker SD, Ten Tije AJ, Carducci MA, Gelderblom H, Dawkins FW, McGuire WP, Verweij J (2004) Evaluation of CYP3A activity as a predictive covariate for docetaxel clearance. J Clin Oncol. ASCO Annual Meeting Proceedings (Post-Meeting Edition). 22: Abstr 2006
Breen KJ, Bury RW, Calder IV, Desmond PV, Peters M, Mashford ML (1984) A [14C]phenacetin breath test to measure hepatic function in man. Hepatology 4:47–52
Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16:187–196
Charles KA, Rivory LP, Stockler MR, Beale P, Beith J, Boyer M, Clarke SJ (2006) Predicting the toxicity of weekly docetaxel in advanced cancer. Clin Pharmacokinet 45:611–622
Danhof M, van Zuilen A, Boeijinga JK, Breimer DD (1982) Studies of the different metabolic pathways of antipyrine in man. Oral versus i.v. administration and the influence of urinary collection time. Eur J Clin Pharmacol 21:433–441
Engel G, Hofmann U, Heidemann H, Cosme J, Eichelbaum M (1996) Antipyrine as a probe for human oxidative drug metabolism: identification of the cytochrome P450 enzymes catalyzing 4-hydroxyantipyrine, 3-hydroxymethylantipyrine, and norantipyrine formation. Clin Pharmacol Ther 59:613–623
Engels FK, Loos WJ, van der Bol JM, de Bruijn P, Mathijssen RH, Verweij J, Mathot RA (2011) Therapeutic drug monitoring for the individualization of docetaxel dosing: a randomized pharmacokinetic study. Clin Cancer Res 17:353–362
Farrell GC, Zaluzny L (1984) Accuracy and clinical utility of simplified tests of antipyrine metabolism. Br J Clin Pharmacol 18:559–565
Felici A, Verweij J, Sparreboom A (2002) Dosing strategies for anticancer drugs: the good, the bad and body-surface area. Eur J Cancer 38:1677–1684
Frassetto LA, Poon S, Tsourounis C, Valera C, Benet LZ (2007) Effects of uptake and efflux transporter inhibition on erythromycin breath test results. Clin Pharmacol Ther 81:828–832
Gartzke J, Jager H (1991) The determination of antipyrine elimination in saliva by liquid chromatography. J Pharm Biomed Anal 9:977–979
Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, Schuetz E, Lim R, Lim HL, Ong AB, Lee HS (2002) Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol 20:3683–3690
Grieco A, Barone C, Coletta P, Castellano R, Ragazzoni E, Cassano A, Astone A, Gambassi G (1992) Antipyrine metabolism in patients with liver metastases from colorectal cancer. Cancer 70:1477–1482
Grieco A, Castellano R, Matera A, Marcoccia S, Di Rocco P, Ragazzoni E, Vecchio FM, Gasbarrini G (1998) Antipyrine clearance in chronic and neoplastic liver diseases: a study of 518 patients. J Gastroenterol Hepatol 13:460–466
Gurney H (1996) Dose calculation of anticancer drugs: a review of the current practice and introduction of an alternative. J Clin Oncol 14:2590–2611
Gustafson DL, Long ME, Zirrolli JA, Duncan MW, Holden SN, Pierson AS, Eckhardt SG (2003) Analysis of docetaxel pharmacokinetics in humans with the inclusion of later sampling time-points afforded by the use of a sensitive tandem LCMS assay. Cancer Chemother Pharmacol 52:159–166
Hilli J, Sailas L, Jyrkkio S, Pyrhonen S, Laine K (2010) NCT01110291: induction of CYP3A activity and lowered exposure to docetaxel in patients with primary breast cancer. Cancer Chemother Pharmacol (Epub ahead of print)
Hirth J, Watkins PB, Strawderman M, Schott A, Bruno R, Baker LH (2000) The effect of an individual’s cytochrome CYP3A4 activity on docetaxel clearance. Clin Cancer Res 6:1255–1258
Hooker AC, Ten Tije AJ, Carducci MA, Weber J, Garrett-Mayer E, Gelderblom H, McGuire WP, Verweij J, Karlsson MO, Baker SD (2008) Population pharmacokinetic model for docetaxel in patients with varying degrees of liver function: incorporating cytochrome P4503A activity measurements. Clin Pharmacol Ther 84:111–118
Hurria A, Fleming MT, Baker SD, Kelly WK, Cutchall K, Panageas K, Caravelli J, Yeung H, Kris MG, Gomez J, Miller VA, D’Andrea G, Scher HI, Norton L, Hudis C (2006) Pharmacokinetics and toxicity of weekly docetaxel in older patients. Clin Cancer Res 12:6100–6105
Kurnik D, Wood AJ, Wilkinson GR (2006) The erythromycin breath test reflects P-glycoprotein function independently of cytochrome P450 3A activity. Clin Pharmacol Ther 80:228–234
Lown K, Kolars J, Turgeon K, Merion R, Wrighton SA, Watkins PB (1992) The erythromycin breath test selectively measures P450IIIA in patients with severe liver disease. Clin Pharmacol Ther 51:229–238
Mahmoud M, Abdel-Kader R, Hassanein M, Saleh S, Botros S (2007) Antipyrine clearance in comparison to conventional liver function tests in hepatitis C virus patients. Eur J Pharmacol 569:222–227
Marre F, Sanderink GJ, de Sousa G, Gaillard C, Martinet M, Rahmani R (1996) Hepatic biotransformation of docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily in humans. Cancer Res 56:1296–1302
Nishio M, Matsuda M, Ohyanagi F, Sato Y, Okumura S, Tabata D, Morikawa A, Nakagawa K, Horai T (2005) Antipyrine test predicts pharmacodynamics in docetaxel and cisplatin combination chemotherapy. Lung Cancer 49:245–251
Nishio MM, M. Karato, A. Sato, Y. Okumura, S. Nakagawa, K. Tabata, D. Morikawa, A, Horai, T (2001) Positive correlation between elimination rate of antipyrine mediated by CYPs and pharmacokinetic parameters of paclitaxel in combination chemotherapy with carboplatin in non-small cell lung cancer patients. Proc Am Soc Clin Oncol 20:445 (Abstr)
O’Reilly S, Rowinsky E, Slichenmyer W, Donehower RC, Forastiere A, Ettinger D, Chen TL, Sartorius S, Bowling K, Smith J, Brubaker A, Lubejko B, Ignacio V, Grochow LB (1996) Phase I and pharmacologic studies of topotecan in patients with impaired hepatic function. J Natl Cancer Inst 88:817–824
Olver I, Davy M, Luftner D, Park SH, Egorin M, Ellis A, Webster L (2001) A phase I study of paclitaxel and altretamine as second-line therapy to cisplatin regimens for ovarian cancer. Cancer Chemother Pharmacol 48:109–114
Orzechowska-Juzwenko K, Wiela A, Cieslinska A, Roszkowska E (1987) Metabolic efficiency of the liver in patients with breast cancer as determined by pharmacokinetics of phenazone. Cancer 59:1607–1610
Preiss R, Matthias M, Sohr R, Brockmann B, Huller H (1987) Pharmacokinetics of adriamycin, adriamycinol, and antipyrine in patients with moderate tumor involvement of the liver. J Cancer Res Clin Oncol 113:593–598
Puisset F, Alexandre J, Treluyer JM, Raoul V, Roche H, Goldwasser F, Chatelut E (2007) Clinical pharmacodynamic factors in docetaxel toxicity. Br J Cancer 97:290–296
Rivory LP, Slaviero KA, Hoskins JM, Clarke SJ (2001) The erythromycin breath test for the prediction of drug clearance. Clin Pharmacokinet 40:151–158
Rizzo JD, Villalona-Calero M, Garrison M, Schwartz G, Molpus K, Monroe P, Tolcher A, Hammond L, Rowinsky EK A pharmacologic and metabolic study of docetaxel (D) administered on a continuous weekly schedule in patients with advanced solid tumors. Proc Am Soc Clin Oncol 22:651 (Abstr)
Schott AF, Rae JM, Griffith KA, Hayes DF, Sterns V, Baker LH (2006) Combination vinorelbine and capecitabine for metastatic breast cancer using a non-body surface area dosing scheme. Cancer Chemother Pharmacol 58:129–135
Schuetz EG, Yasuda K, Arimori K, Schuetz JD (1998) Human MDR1 and mouse mdr1a P-glycoprotein alter the cellular retention and disposition of erythromycin, but not of retinoic acid or benzo(a)pyrene. Arch Biochem Biophys 350:340–347
Slaviero KA, Clarke SJ, McLachlan AJ, Blair EY, Rivory LP (2004) Population pharmacokinetics of weekly docetaxel in patients with advanced cancer. Br J Clin Pharmacol 57:44–53
Tanaka E, Breimer DD (1997) In vivo function tests of hepatic drug-oxidizing capacity in patients with liver disease. J Clin Pharm Ther 22:237–249
ten Tije AJ, Verweij J, Carducci MA, Graveland W, Rogers T, Pronk T, Verbruggen MP, Dawkins F, Baker SD (2005) Prospective evaluation of the pharmacokinetics and toxicity profile of docetaxel in the elderly. J Clin Oncol 23:1070–1077
Tran A, Jullien V, Alexandre J, Rey E, Rabillon F, Girre V, Dieras V, Pons G, Goldwasser F, Treluyer JM (2006) Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther 79:570–580
Watkins PB, Murray SA, Winkelman LG, Heuman DM, Wrighton SA, Guzelian PS (1989) Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. Studies in rats and patients. J Clin Invest 83:688–697
Webster LK, Ellis AG, Bishop JF (1993) Effect of toremifene on antipyrine elimination in the isolated perfused rat liver. Cancer Chemother Pharmacol 31:319–323
Winchell HS, Stahelin H, Kusubov N, Slanger B, Fish M, Pollycove M, Lawrence JH (1970) Kinetics of CO2-HCO3 minus in normal adult males. J Nucl Med 11:711–715
Yamamoto N, Tamura T, Kamiya Y, Sekine I, Kunitoh H, Saijo N (2000) Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. J Clin Oncol 18:2301–2308
Conflict of interest
The listed authors have no conflict of interests. Partial funding by Cancer Trials Australia
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michael, M., Cullinane, C., Hatzimihalis, A. et al. Docetaxel pharmacokinetics and its correlation with two in vivo probes for cytochrome P450 enzymes: the C14-erythromycin breath test and the antipyrine clearance test. Cancer Chemother Pharmacol 69, 125–135 (2012). https://doi.org/10.1007/s00280-011-1676-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1676-y