Abstract
Purpose
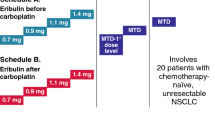
To determine the maximum tolerated dose (MTD), safety and tolerability of sunitinib plus pemetrexed and cisplatin for advanced solid malignancies.
Methods
Using a 3 + 3 dose-escalation design, patients received oral sunitinib (37.5 or 50 mg) qd on a continuous daily dosing (CDD) schedule or Schedule 2/1 (2 weeks on, 1 week off treatment) plus pemetrexed (400 or 500 mg/m2 IV) and cisplatin (75 mg/m2 IV) q3w up to 6 cycles.
Results
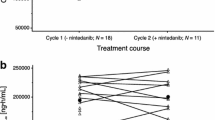
Sunitinib 37.5 mg/pemetrexed 400 mg/m2/cisplatin 75 mg/m2 CDD (n = 5) was not tolerated. Lower doses on this schedule were not explored. The Schedule 2/1 MTD (n = 15) was sunitinib 37.5 mg/pemetrexed 500 mg/m2/cisplatin 75 mg/m2, based on one dose-limiting toxicity (myocardial infarction) out of six patients. The MTD was further studied in an expansion cohort of 10 non-small cell lung cancer (NSCLC) patients and one mesothelioma patient. There were no clinically significant drug–drug interactions. Cumulative myelosuppression was problematic: the median relative dose intensity (% actual/intended) across all cycles was 61 % for sunitinib, 78 % for pemetrexed, and 74 % for cisplatin. Four of eight NSCLC patients in the dose-escalation and expansion cohorts at the Schedule 2/1 MTD who were evaluable for efficacy had stable disease ≥8 weeks, and the one patient with mesothelioma had a partial response.
Conclusions
In patients with advanced solid malignancies, sunitinib was not tolerated at 37.5 mg CDD with standard pemetrexed and cisplatin doses. Dose reductions were often needed due to cumulative myelosuppression following cycle 1. The MTD showed modest antitumor activity.

Similar content being viewed by others
References
Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM (2003) SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2:471–478
Chow LQ, Eckhardt SG (2007) Sunitinib: from rational design to clinical efficacy. J Clin Oncol 25:884–896
Kim DW, Jo YS, Jung HS, Chung HK, Song JH, Park KC, Park SH, Hwang JH, Rha SY, Kweon GR, Lee SJ, Jo KW, Shong M (2006) An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab 91:4070–4076
Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9:327–337
Murray LJ, Abrams TJ, Long KR, Ngai TJ, Olson LM, Hong W, Keast PK, Brassard JA, O’Farrell AM, Cherrington JM, Pryer NK (2003) SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis 20:757–766
O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM (2003) SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 101:3597–3605
Figg WD, Folkman J (eds) (2008) Angiogenesis: an integrative approach from science to medicine. Springer, New York
Hasumi Y, Klosowska-Wardega A, Furuhashi M, Ostman A, Heldin CH, Hellberg C (2007) Identification of a subset of pericytes that respond to combination therapy targeting PDGF and VEGF signaling. Int J Cancer 121:2606–2614
Koukourakis MI, Giatromanolaki A, O’Byrne KJ, Comley M, Whitehouse RM, Talbot DC, Gatter KC, Harris AL (1997) Platelet-derived endothelial cell growth factor expression correlates with tumour angiogenesis and prognosis in non-small-cell lung cancer. Br J Cancer 75:477–481
Shikada Y, Yonemitsu Y, Koga T, Onimaru M, Nakano T, Okano S, Sata S, Nakagawa K, Yoshino I, Maehara Y, Sueishi K (2005) Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res 65:7241–7248
Yuan A, Yu CJ, Kuo SH, Chen WJ, Lin FY, Luh KT, Yang PC, Lee YC (2001) Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J Clin Oncol 19:432–441
SUTENT PI. SUTENT, Summary of Product Characteristics, Pfizer Inc. 2010
Novello S, Scagliotti GV, Rosell R, Socinski MA, Brahmer J, Atkins J, Pallares C, Burgess R, Tye L, Selaru P, Wang E, Chao R, Govindan R (2009) Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer 101:1543–1548
Raymond E, Dahan L, Raoul J-L, Bang Y-J, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364:501–513
Rosen LS, Bello CL, Mulay M, Dinolfo M, Baum C (2006) A phase I study evaluating administration of oral SU11248 (sunitinib malate) using a loading and maintenance dose on a 2/1 schedule in patients (pts) with advanced solid tumors. In: Proceedings of the 97th American Association for Cancer Research 47; abstract 2911
Socinski MA, Novello S, Brahmer JR, Rosell R, Sanchez JM, Belani CP, Govindan R, Atkins JN, Gillenwater HH, Pallares C, Tye L, Selaru P, Chao RC, Scagliotti GV (2008) Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol 26:650–656
Escudier B, Roigas J, Gillessen S, Harmenberg U, Srinivas S, Mulder SF, Fountzilas G, Peschel C, Flodgren P, Maneval EC, Chen I, Vogelzang NJ (2009) Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol 27:4068–4075
George S, Blay JY, Casali PG, Le CA, Stephenson P, DePrimo SE, Harmon CS, Law CN, Morgan JA, Ray-Coquard I, Tassell V, Cohen DP, Demetri GD (2009) Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 45:1959–1968
Motzer RJ, Michaelson MD, Rosenberg J, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Wilding G (2007) Sunitinib efficacy against advanced renal cell carcinoma. J Urol 178:1883–1887
Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WJ, Wilding G, Martell B, Hariharan S, Figlin RA (2011) Randomized phase II multicenter study of the efficacy and safety of sunitinib on the 4/2 versus continuous dosing schedule as first-line therapy of metastatic renal cell carcinoma: renal EFFECT Trial. J Clin Oncol 29(Suppl 7); abstract LBA308
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543–3551
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
Chow LQ, Blais N, Jonker DJ, Laurie SA, Diab SG, Canil C, McWilliam M, Thall A, Ruiz-Garcia A, Zhang K, Tye L, Chao RC, Camidge DR (2011) A phase I dose-escalation and pharmacokinetic study of sunitinib in combination with pemetrexed in patients with advanced solid malignancies, with an expanded cohort in non-small cell lung cancer. Cancer Chemother Pharmacol Oct 12 (e-pub ahead of print)
Reck M, Frickhofen N, Cedres S, Gatzemeier U, Heigener D, Fuhr H-G, Thall A, Lanzalone S, Stephenson P, Ruiz-Garcia A, Chao R, Felip E (2010) Sunitinib in combination with gemcitabine plus cisplatin for advanced non-small cell lung cancer: a phase I dose-escalation study. Lung Cancer 70:180–187
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Byrne MJ, Nowak AK (2004) Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 15:257–260
Zhang GZ, Jiao SC, Meng ZT (2010) Pemetrexed plus cisplatin/carboplatin in previously treated locally advanced or metastatic non-small cell lung cancer patients. J Exp Clin Cancer Res 29:38
Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD (2008) Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 26:1810–1816
Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WJ, Wilding G, Martell B, Hariharan S, Figlin RA (2011) Randomized phase II multicenter study of the efficacy and safety of sunitinib on the 4/2 versus continuous dosing schedule as first-line therapy of metastatic renal cell carcinoma: renal EFFECT Trial. J Clin Oncol 29(suppl 7); abstract LBA308
Laurie SA, Gupta A, Chu Q, Lee CW, Morzycki W, Feld R, Foo AH, Seely J, Goffin JR, Laberge F, Murray N, Rao S, Nicholas G, Laskin J, Reiman T, Sauciuc D, Seymour L (2011) Brief Report: a phase II study of sunitinib in malignant pleural mesothelioma. The NCIC Clinical Trials Group. J Thorac Oncol 6:1950–1954
Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, Abrão Miziara JE, Balint B, De Marinis F, Keller A, Arén O, Csollak M, Albert I, Barrios CH, Grossi F, Krzakowski M, Cupit L, Cihon F, Dimatteo S, Hanna N (2010) Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 28:1835–1842
Camidge DR, Kono SA, Lu X, Okuyama S, Barón AE, Oton AB, Davies AM, Varella-Garcia M, Franklin W, Doebele RC (2011) Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol 6:774–780
Acknowledgments
We would like to thank all of the participating patients and their families, as well as the global network of investigators, research nurses, study coordinators, and operations staff. We would also like to acknowledge Tanya Boutros for coordination of PK assays, and Michelle McWilliam for data review. Medical writing support was provided by Susanne Gilbert at ACUMED (New York, NY) and was funded by Pfizer Inc. This study was sponsored by Pfizer Inc.
Conflict of interest
A Ruiz-Garcia, A Thall, K Zhang, and RC Chao are employees of Pfizer Inc. and hold stock in Pfizer Inc., the makers of SUTENT®. RC Doebele has research grants from ImClone Systems, Inc., Eli Lilly & Co., and Pfizer, Inc.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Camidge, D.R., Blais, N., Jonker, D.J. et al. Sunitinib combined with pemetrexed and cisplatin: results of a phase I dose-escalation and pharmacokinetic study in patients with advanced solid malignancies, with an expanded cohort in non-small cell lung cancer and mesothelioma. Cancer Chemother Pharmacol 71, 307–319 (2013). https://doi.org/10.1007/s00280-012-2008-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-2008-6