Abstract
Purpose
The aim of our study was to investigate the efficacy and safety of pemetrexed monotherapy in chemo-naïve Eastern Cooperative Oncology Group (ECOG) performance status (PS) 2 patients with epidermal growth factor receptor (EGFR) wild-type or unknown advanced non-squamous non-small cell lung cancer (NSCLC).
Methods
Pemetrexed was administered at 500 mg/m2 triweekly until progression with supplementations in chemo-naïve ECOG PS 2 patients with EGFR wild-type or unknown advanced non-squamous NSCLC.
Results
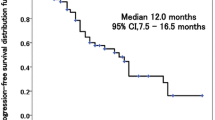
Between September 2009 and April 2013, twenty-eight patients were enrolled. Median age was 75 (range 59–89). Nineteen (68 %) of 28 were ever smoker, and 18 (64 %) had pulmonary emphysema. Sixteen (57 %) had comorbidities such as hypertension, heart disease, and/or diabetes. In 26 eligible patients, the overall response rate, disease control rate, median PFS, and median overall survival were 11.5, 53.8 %, 3.0 [95 % confidence interval (CI) 1.9–5.7] months and 9.5 (95 % CI 3.3–12.5) months, respectively. Median administered course number was 3 (range 1–14). Median duration of PS maintenance ≤2 was 4.9 (95 % CI 1.3–9.7) months. Common (≥10 %) grade 3/4 toxicities included 7 (27 %) neutropenia, 7 (27 %) leukopenia, 4 (15 %) fatigue, and 3 (12 %) thrombocytopenia. Febrile neutropenia and interstitial lung disease were not observed. There were no treatment-related deaths.
Conclusion
Pemetrexed monotherapy demonstrated moderate efficacy and good safety in chemo-naïve PS 2 patients with EGFR wild-type or unknown non-squamous NSCLC. It can be a therapeutic option in “frail” PS 2 non-squamous NSCLC patients without the indication of combination regimens, if the patient is EGFR wild-type.



Similar content being viewed by others
References
Lilenbaum RC, Cashy J, Hensing TA et al (2008) Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol 3:125–129
Kawaguchi T, Takada M, Kubo A et al (2010) Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 5:620–630
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Soda M, Choi YL, Enomoto M et al (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448:561–566
Inoue A, Kobayashi K, Usui K et al (2009) First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 27:1394–1400
Lilenbaum R, Axelrod R, Thomas S et al (2008) Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 26:863–869
Zukin M, Barrios CH, Pereira JR et al (2013) Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 31:2849–2853
Scagliotti G, Hanna N, Fossella F et al (2009) The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist 14:253–263
Hanna N, Shepherd FA, Fossella FV et al (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589–1597
Ohe Y, Ichinose Y, Nakagawa K et al (2008) Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin Cancer Res 14:4206–4212
National Comprehensive Cancer Network (2015) NCCN clinical practice guidelines in oncology—non-small cell lung cancer, version 3.2015. http://www.nccn.org
Langer C, V S, Schiller J, Eastern Cooperative Oncology Group et al (2007) Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in Eastern Cooperative Oncology Group performance status 2 non-small-cell lung cancer patients: ECOG 1599. J Clin Oncol 25:418–423
Langer CJ, O’Byrne KJ, Socinski MA et al (2008) Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naïve advanced non-small cell lung cancer. J Thorac Oncol 3:623–630
O’Brien ME, Socinski MA, Popovich AY et al (2008) Randomized phase III trial comparing single-agent paclitaxel poliglumex (CT-2103, PPX) with single-agent gemcitabine or vinorelbine for the treatment of PS 2 patients with chemotherapy-naïve advanced non-small cell lung cancer. J Thorac Oncol 3:728–734
Saito H, Nakagawa K, Takeda K et al (2012) Randomized phase II study of carboplatin–paclitaxel or gemcitabine–vinorelbine in patients with advanced nonsmall cell lung cancer and a performance status of 2: West Japan Thoracic Oncology Group 0004. Am J Clin Oncol 35:58–63
Kosmidis PA, Dimopoulos MA, Syrigos K et al (2007) Gemcitabine versus gemcitabine–carboplatin for patients with advanced non-small cell lung cancer and a performance status of 2: a prospective randomized phase II study of the Hellenic Cooperative Oncology Group. J Thorac Oncol 2:135–140
Lilenbaum RC, Herndon JE II, List MA et al (2005) Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the Cancer and Leukemia Group B (study 9730). J Clin Oncol 23:190–196
Lilenbaum R, Villaflor VM, Langer C et al (2009) Single-agent versus combination chemotherapy in patients with advanced non-small cell lung cancer and a performance status of 2: prognostic factors and treatment selection based on two large randomized clinical trials. J Thorac Oncol 4:869–874
Azzoli CG, Baker S Jr, Temin S et al (2009) American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 27:6251–6266
Peters S, V AA, Gridelli C, ESMO Guidelines Working Group et al (2012) Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):56–64
Acknowledgments
This study was supported by Foundation for Biomedical Research and Innovation.
Conflict of interest
Akito Hata, Nobuyuki Katakami, and Satoshi Morita received a lecture fee from Eli Lilly. The other authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hata, A., Katakami, N., Fujita, S. et al. A phase II study of pemetrexed monotherapy in chemo-naïve Eastern Cooperative Oncology Group performance status 2 patients with EGFR wild-type or unknown advanced non-squamous non-small cell lung cancer (HANSHIN Oncology Group 002). Cancer Chemother Pharmacol 75, 1267–1272 (2015). https://doi.org/10.1007/s00280-015-2755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2755-2