Abstract
Purpose
Pomalidomide is a distinct immunomodulatory agent approved for the treatment of relapsed and refractory multiple myeloma. QT interval was monitored in prior studies, and although there was no indication that pomalidomide could induce QT prolongation, a formal analysis was not previously conducted. Therefore, we present here the results of a study evaluating the effects of pomalidomide on QT interval corrected for heart rate (QTc) using Fridericia’s formula (QTcF) and other electrocardiogram (ECG) parameters.
Methods
Healthy male volunteers with normal or clinically acceptable laboratory tests and ECG results were randomized to receive single oral doses of placebo, pomalidomide 4 mg, pomalidomide 20 mg, and moxifloxacin 400 mg (positive control) in different sequences. Subjects were evaluated by digital ECG monitoring and underwent blood sampling and standard safety monitoring.
Results
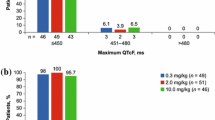
Seventy-two subjects were enrolled. In ECG evaluations performed after dosing with pomalidomide 4 mg (therapeutic dose) or 20 mg (supratherapeutic dose), the upper limit of the two-sided 90 % CI for mean change from baseline and placebo-corrected QTcF was <10 ms at all postdose time points, which is below the defined threshold of regulatory concern. In contrast, moxifloxacin induced a clear prolongation in QT interval. Changes in heart rate and other ECG parameters after pomalidomide dosing were clinically insignificant.
Conclusions
Pomalidomide given as a single oral dose of up to 20 mg was not associated with QT prolongation in healthy male subjects.



Similar content being viewed by others
References
Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, Prince HM (2010) Mechanism of action of immunomodulatory drugs (IMiDs) in multiple myeloma. Leukemia 24:22–32. doi:10.1038/leu.2009.236
Imnovid (pomalidomide) (2015) Summary of product characteristics. Celgene Europe Ltd., Uxbridge
Pomalyst (pomalidomide) (2015) Package insert. Celgene Corporation, Summit
Richardson PG, Siegel DS, Vij R et al (2014) Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood 123:1826–1832. doi:10.1182/blood-2013-11-538835
San Miguel J, Weisel K, Moreau P et al (2013) Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 14:1055–1066. doi:10.1016/S1470-2045(13)70380-2
Hoffmann M, Kasserra C, Reyes J, Schafer P, Kosek J, Capone L, Parton A, Kim-Kang H, Surapaneni S, Kumar G (2013) Absorption, metabolism and excretion of [14C]pomalidomide in humans following oral administration. Cancer Chemother Pharmacol 71:489–501. doi:10.1007/s00280-012-2040-6
Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, Streetly M, Dalgleish AG (2004) Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol 22:3269–3276. doi:10.1200/JCO.2004.10.052
Kasserra C, Assaf M, Hoffmann M, Li Y, Liu L, Wang X, Kumar G, Palmisano M (2015) Pomalidomide: evaluation of cytochrome P450 and transporter-mediated drug–drug interaction potential in vitro and in healthy subjects. J Clin Pharmacol 55:168–178. doi:10.1002/jcph.384
Vigneault P, Kaddar N, Bourgault S, Caillier B, Pilote S, Patoine D, Simard C, Drolet B (2011) Prolongation of cardiac ventricular repolarization under paliperidone: how and how much? J Cardiovasc Pharmacol 57:690–695. doi:10.1097/FJC.0b013e318217d941
Rajamani S, Eckhardt LL, Valdivia CR, Klemens CA, Gillman BM, Anderson CL, Holzem KM, Delisle BP, Anson BD, Makielski JC, January CT (2006) Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br J Pharmacol 149:481–489. doi:10.1038/sj.bjp.0706892
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2005) ICH harmonised tripartite guideline: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. Accessed 2 Sept 2015
US Food and Drug Administration: Center for Drug Evaluation and Research. POMALYST (pomalidomide) NDA 204026. Pharmacology/toxicology NDA review and evaluation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204026Orig1s000PharmR.pdf. Accessed 2 Sept 2015
Chen N, Ye Y, Liu L, Reyes J, Assaf MS, Kasserra C, Zhou S, Palmisano M (2013) Lenalidomide at therapeutic and supratherapeutic doses does not prolong QTc intervals in the thorough QTc study conducted in healthy men. Basic Clin Pharmacol Toxicol 113:179–186. doi:10.1111/bcpt.12081
Bloomfield DM, Kost JT, Ghosh K, Hreniuk D, Hickey LA, Guitierrez MJ, Gottesdiener K, Wagner JA (2008) The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther 84:475–480
Fridericia LS (1920) Die systolendauer im elektrokardiogramm bei normalen menschen und bei Herzkranken. Acta Med Scand 53:469–486
Food and Drug Administration (2005) Guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073153.pdf. Accessed 4 Sept 2015
Acknowledgments
Editorial assistance was provided MediTech Media, supported by Celgene.
Funding
This study was supported by Celgene Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.M. is an employee of PPD Phase 1 Clinic; M.A. and E.O. are employees of and have stock ownership in Celgene Corporation; L.L. is an employee of Celgene Corporation.
Rights and permissions
About this article
Cite this article
Mondal, S.A., Assaf, M., Liu, L. et al. A Phase 1, double-blind, 4-period crossover study to investigate the effects of pomalidomide on QT interval in healthy male subjects. Cancer Chemother Pharmacol 77, 251–258 (2016). https://doi.org/10.1007/s00280-015-2912-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2912-7