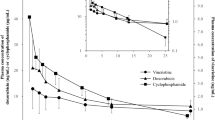
Abstract
Purpose
For many cancers, adolescents and young adults (AYA) have worse outcomes than for children and adults. Many factors may contribute to the AYA survival gap, including differences in biology, therapeutic intent, and adherence to therapy. It has been observed that male AYAs have poorer outcomes than females. The purpose of this work was to test the proposition that gender-related pharmacologic factors may account for a component of the AYA survival gap.
Patients and methods
A prospective, multi-institutional pharmacologic study of 79 patients in total with chemosensitive cancers (Ewing sarcoma, osteosarcoma and Hodgkin lymphoma) was conducted, with conventional doxorubicin treatment. Pharmacokinetic data of 13 children, 40 AYAs and 13 adults were valid for analysis. Population pharmacokinetics models were developed for doxorubicin and its metabolite doxorubicinol based on the data created in this study. Consequently, model-based analysis was conducted to investigate the relevant topics.
Results
The clearance of doxorubicinol (normalized to body surface area), the main active metabolite of doxorubicin, appears faster in male AYAs than female (p = 0.04, 95% CI 0.1–3.9 L/h). The exposure of doxorubicinol (normalized to dose) is lower in male AYA than female (p = 0.03, 95% CI − 0.005 to − 0.0002 h/L). These might be correlated to the observed difference on nadir neutrophil count between male AYA and female (p = 0.027, 95% CI 0.09–1.4).
Conclusion
Gender-related differences in doxorubicin pharmacology may account for worse outcomes for male AYAs with chemosensitive cancers compared to females. These findings may reduce the AYA survival gap compared to other age groups.












Similar content being viewed by others
References
Bleyer A, Viny A, Barr R (2006) Cancer in 15- to 29-year-olds by primary site. Oncologist 11:590–601
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43–66
Albritton K, Bleyer WA (2003) The management of cancer in the older adolescent. Eur J Cancer 39:2584–2599
Bleyer A (2002) Older adolescents with cancer in North America deficits in outcome and research. Pediatr Clin North Am 49:1027–1042
Bleyer A (2005) The adolescent and young adult gap in cancer care and outcome. Curr Probl Pediatr Adolesc Health Care 35:182–217
Mitchell AE et al (2004) Cancer in adolescents and young adults: treatment and outcome in Victoria. Med J Aust 180:59–62
Khamly KK, Thursfield VJ, Fay M, Desai J, Toner GC, Choong PF, Ngan SY, Powell GJ, Thomas DM (2009) Gender-specific activity of chemotherapy correlates with outcomes in chemosensitive cancers of young adulthood. Int J Cancer 125:426–431
Klimm B, Reineke T, Haverkamp H, Behringer K, Eich HT, Josting A, Pfistner B, Diehl V, Engert A (2005) Role of hematotoxicity and sex in patients with Hodgkin’s lymphoma: an analysis from the German Hodgkin Study Group. J Clin Oncol 23:8003–8011
Bacci G, Longhi A, Ferrari S, Mercuri M, Versari M, Bertoni F (2006) Prognostic factors in non-metastatic Ewing’s sarcoma tumor of bone: an analysis of 579 patients treated at a single institution with adjuvant or neoadjuvant chemotherapy between 1972 and 1998. Acta Oncol 45:469–475
Gehan EA, Nesbit ME Jr, Burgert EO Jr, Viettit J, Tefft M, Perez CA, Kissane J, Hempel C. (1981) Prognostic factors in children with Ewing’s sarcoma. Natl Cancer Inst Monogr 56:273–278
Collins M et al (2013) Benefits and adverse events in younger versus older patients receiving neoadjuvant chemotherapy for osteosarcoma: findings from a meta-analysis. J Clin Oncol 31:2303–2312
Smeland S, Muller C, Alvegard TA, Wiklund T, Wiebe T, Bjork O, Stenwig AE, Willen H, Holmstrom T, Folleras G, Brosjo O, Kivioja A et al (2003) Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer 39:488–494
Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H et al (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20:776–790
Martin JH, Phillips E, Thomas DM, Somogyi A (2015) Adding the ‘medicines’ back in personaolized medicine to improve cancer treatment outcomes. Br J Clin Pharmacol 80:929–931
Kansara M, Teng W, Smyth M, Thomas DM (2014) Translational Biology of osteosarcoma. Nat Rev Cancer 14:722–735
Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, Delattre O, Paulussen M, Picci P, Sundby Hall K, van den Berg H, Ladenstein R, Michon J, Hjorth L, Judson I, Luksch R, Bernstein ML, Marec-Bérard P, Brennan B, Craft AW, Womer RB, Juergens H, Oberlin O (2015) Ewing sarcoma: current management and future approaches through collaboration. J Clin Oncol 33:3036–3046
Kahn JM, Kelly KM (2018) Adolescent and young adult Hodgkin lymphoma: raising the bar through collaborative science and multidisciplinary care. Pediatr Blood Cancer 30:e27033
Gewirtz DA (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57:727–741
Boucek RJ, Olson RD, Brenner DE, Ogunbunmi EM, Inui M, Fleischer S (1987) The major metabolite of doxorubicin is a potent inhibitor of membrane-associated ion pumps. A correlative study of cardiac muscle with isolated membrane fractions. J Biol Chem 262:15851–15856
Gilbert CM, McGeary RP, Filippich LJ, Norris RL, Charles BG (2005) Simultaneous liquid chromatographic determination of doxorubicin and its major metabolite doxorubicinol in parrot plasma. J Chromatogr B Anal Technol Biomed Life Sci 826(1–2):273–276
Beal SSL, Boekmann A, Bauer RJ (2009) NONMEM’s user’s guides. ICON Development Solutions, Ellicott City
Lindbom L, Pihlgren P, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)--a Perl module for NONMEM related programming. Comput Methods Progr Biomed 75(2):85–94
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Progr Biomed 79(3):241–257
http://www.pltsoft.com. Accessed 10 Sept 2018
R_Core_Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Kontny NE, Wurthwein G, Joachim B, Boddy AV, Krischke M, Fuhr U, Thompson PA, Jorger M, Schellens JH, Hempel G (2013) Population pharmacokinetics of doxorubicin: establishment of a NONMEM model for adults and children older than 3 years. Cancer Chemother Pharmacol 71(3):749–763. https://doi.org/10.1007/s00280-013-2069-1
Voller S, Boos J, Krischke M, Wurthwein G, Kontny NE, Boddy AV, Hempel G (2015) Age-dependent pharmacokinetics of doxorubicin in children with cancer. Clin Pharmacokinet. https://doi.org/10.1007/s40262-015-0272-4
Frost BM, Eksborg S, Bjork O, Abrahamsson J, Behrendtz M, Castor A, Forestier E, Lonnerholm G (2002) Pharmacokinetics of doxorubicin in children with acute lymphoblastic leukemia: multi-institutional collaborative study. Med Pediatr Oncol 38(5):329–337. https://doi.org/10.1002/mpo.10052
Kunarajah K, Hennig S, Norris RL, Lobb M, Charles BG, Pinkerton R, Moore AS (2017) Population pharmacokinetic modelling of doxorubicin and doxorubicinol in children with cancer: is there a relationship with cardiac troponin profiles?. Cancer Chemother Pharmacol 80:15–25
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28(5):481–504
https://uupharmacometrics.github.io/PsN/docs.html. Accessed Aug 2018
Parke J, Holford NH, Charles BG (1999) A procedure for generating bootstrap samples for the validation of nonlinear mixedeffects population models. Comput Methods Progr Biomed 59(1):19–29
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J 532 Pharmacokinet Pharmacodyn 28:481–504
Pérez-Blanco JS, Santos-Buelga D, Fernández De Gatta MDM et al (2016) Population pharmacokinetic of doxorubicin and doxorubicinol in patients diagnosed with non-Hodgkin´s lymphoma. Br J Clin Pharmacol 82:1517–1527
Callies S, de Alwis DP, Wright JG, Sandler A, Burgess M, Aarons L (2003) A population pharmacokinetic model for doxorubicin and doxorubicinol in the presence of a novel MDR modulator, zosuquidar trihydrochloride (LY335979). Cancer Chemother Pharmacol 51:107–118
Veal GJ, Hartford CM, Stewart CF (2010) Clinical pharmacology in the adolescent oncology patient. J Clin Oncol 28:4790–4799
Cusack BJ, Young SP, Driskell J, Olson RD (1993) Doxorubicin and doxorubicinol pharmacokinetics and tissue concentrations following bolus injection and continuous infusion of doxorubicin in the rabbit. Cancer Chemother Pharmacol 32:53–58
Acknowledgements
Funding was provided by Victorian Cancer Agency (Grant no. CTPS_08_18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Appendices
Appendix 1
See Table 3.
Appendix 2
During the modelling process, the fraction (Fm, in Fig. 2) of doxorubicin metabolized to doxorubicinol was fixed as 22% [34]. The parameter value of Q4 was calculated as 22% of CLDoxo (39.0 L/h/1.8 m2 in Table 2). The reason of fixing Fm is for parameter identifiability purpose. In the model, the metabolite volume (V4) is dependent on the amount of doxorubicinol compared to the concentration of doxorubicinol and the amount of doxorubicinol is dependent on the fractional of doxorubicin drug metabolized (Fm). V4 and Fm cannot be identified simultaneously in the model.
In similar recent studies of modelling doxorubicin and its metabolite, two methods were applied to bypass the issue of parameter identifiability. One way was to fix directly the value of V4 [34], the other way was to fix Fm or Q4 [26, 29]. Of note, the sources of the fixed values of Fm or Q4 were not elucidated in Kontny and Kunarajah’s work, and the fixed values appear rather arbitrary. In our study, we fixed the value of Fm as estimated in Pérez-Blanco’s work. Consequently, the parameter values estimated in this study (i.e. CLDox’ol and V4) depend on the fixed value of Fm and are apparent values.
Worthwhile to notice that the value of Fm fixed in our study is an apparent, not absolute value. The fixed fraction Fm (no matter is 22% or other values) would not undermine our conclusion (i.e. clearance of dox’ol is faster in male AYA than female). This is because different fraction values used only change the clearance proportionally.
Appendix 3
Prediction-corrected and variance-corrected VPCs for doxorubicin for AYA patients. The raw data are represented as black circles and lines. Grey shaded areas are the 90% confidence interval for the 5th, 50th and 95th percentiles of the simulated data. Four bins are used and the short yellow bars are the bin boundaries
Prediction-corrected and variance-corrected VPCs for doxorubicinol for AYA patients. The raw data are represented as black circles and lines. Grey shaded areas are the 90% confidence interval for the 5th, 50th and 95th percentiles of the simulated data. Four bins are used and the short yellow bars are the bin boundaries
Rights and permissions
About this article
Cite this article
Liu, Z., Martin, J., Orme, L. et al. Gender differences in doxorubicin pharmacology for subjects with chemosensitive cancers of young adulthood. Cancer Chemother Pharmacol 82, 887–898 (2018). https://doi.org/10.1007/s00280-018-3683-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3683-8