Abstract
Purpose
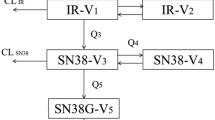
Irinotecan (IR) displays significant PK/PD variability. This study evaluated functional hepatic imaging (HNI) and extensive pharmacogenomics (PGs) to explore associations with IR PK and PD (toxicity and response).
Methods
Eligible patients (pts) suitable for Irinotecan-based therapy. At baseline: (i) PGs: blood analyzed by the Affymetrix-DMET™-Plus-Array (1936 variants: 1931 single nucleotide polymorphisms [SNPs] and 5 copy number variants in 225 genes, including 47 phase I, 80 phase II enzymes, and membrane transporters) and Sanger sequencing (variants in HNF1A, Topo-1, XRCC1, PARP1, TDP, CDC45L, NKFB1, and MTHFR), (ii) HNI: pts given IV 250 MBq-99mTc-IDA, data derived for hepatic extraction/excretion parameters (CLHNI, T1/2-HNI, 1hRET, HEF, Td1/2). In cycle 1, blood was taken for IR analysis and PK parameters were derived by non-compartmental methods. Associations were evaluated between HNI and PGs, with IR PK, toxicity, objective response rate (ORR) and progression-free survival (PFS).
Results
N = 31 pts. The two most significant associations between PK and PD with gene variants or HNI parameters (P < 0.05) included: (1) PK: SN38-Metabolic Ratio with CLHNI, 1hRET, (2) Grade 3+ diarrhea with SLC22A2 (rs 316019), GSTM5 (rs 1296954), (3) Grade 3+ neutropenia with CLHNI, 1hRET, SLC22A2 (rs 316019), CYP4F2 (rs2074900) (4) ORR with ALDH2 (rs 886205), MTHFR (rs 1801133). (5) PFS with T1/2-HNI, XDH (rs 207440), and ABCB11 (rs 4148777).
Conclusions
Exploratory associations were observed between Irinotecan PK/PD with hepatic functional imaging and extensive pharmacogenomics. Further work is required to confirm and validate these findings in a larger cohort of patients.
Australian New Zealand Clinical Trials Registry (ANZCTR) Number
ACTRN12610000897066, Date registered: 21/10/2010.
Similar content being viewed by others
References
Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A (2001) Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 7(8):2182–2194
Chu XY, Kato Y, Ueda K, Suzuki H, Niinuma K, Tyson CA, Weizer V, Dabbs JE, Froehlich R, Green CE, Sugiyama Y (1998) Biliary excretion mechanism of CPT-11 and its metabolites in humans: involvement of primary active transporters. Cancer Res 58(22):5137–5143
Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I (2005) Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos 33(3):434–439. https://doi.org/10.1124/dmd.104.001909
Schellens JH, Maliepaard M, Scheper RJ, Scheffer GL, Jonker JW, Smit JW, Beijnen JH, Schinkel AH (2000) Transport of topoisomerase I inhibitors by the breast cancer resistance protein. Potential clinical implications. Ann N Y Acad Sci 922:188–194
de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S (2018) Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet. https://doi.org/10.1007/s40262-018-0644-7
Sugatani J, Sueyoshi T, Negishi M, Miwa M (2005) Regulation of the human UGT1A1 gene by nuclear receptors constitutive active/androstane receptor, pregnane X receptor, and glucocorticoid receptor. Methods Enzymol 400:92–104. https://doi.org/10.1016/S0076-6879(05)00006-6
van der Bol JM, Mathijssen RH, Loos WJ, Friberg LE, van Schaik RH, de Jonge MJ, Planting AS, Verweij J, Sparreboom A, de Jong FA (2007) Cigarette smoking and irinotecan treatment: pharmacokinetic interaction and effects on neutropenia. J Clin Oncol 25(19):2719–2726. https://doi.org/10.1200/JCO.2006.09.6115
Mathijssen RH, Verweij J, de Jonge MJ, Nooter K, Stoter G, Sparreboom A (2002) Impact of body-size measures on irinotecan clearance: alternative dosing recommendations. J Clin Oncol 20(1):81–87. https://doi.org/10.1200/JCO.2002.20.1.81
Hahn RZ, Antunes MV, Verza SG, Perassolo MS, Suyenaga ES, Schwartsmann G, Linden R (2018) Pharmacokinetic and pharmacogenetic markers of irinotecan toxicity. Curr Med Chem. https://doi.org/10.2174/0929867325666180622141101
US Food and Drug Administration (2014) Irinotecan hydrochloride. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e98886aa-933c-430f-bb56-f1eb3862aae4#boxedwarning
Association RDP (2014) Dutch Pharmacogenetics Working Group (DPWG). Pharmacogenetic Guidelines. Netherlands. Irinotecan-UGT1A1. http://kennisbank.knmp.nl
Michael M, Thompson M, Hicks RJ, Mitchell PL, Ellis A, Milner AD, Di Iulio J, Scott AM, Gurtler V, Hoskins JM, Clarke SJ, Tebbut NC, Foo K, Jefford M, Zalcberg JR (2006) Relationship of hepatic functional imaging to irinotecan pharmacokinetics and genetic parameters of drug elimination. J Clin Oncol 24(26):4228–4235. https://doi.org/10.1200/JCO.2005.04.8496
Affymetrix I (2008) DMETTM plus array. https://assets.thermofisher.com/TFS-Assets/LSG/manuals/dmet_plus_array_insert.pdf
Deeken J (2009) The Affymetrix DMET platform and pharmacogenetics in drug development. Curr Opin Mol Ther 11(3):260–268
Horita Y, Yamada Y, Hirashima Y, Kato K, Nakajima T, Hamaguchi T, Shimada Y (2010) Effects of bevacizumab on plasma concentration of irinotecan and its metabolites in advanced colorectal cancer patients receiving FOLFIRI with bevacizumab as second-line chemotherapy. Cancer Chemother Pharmacol 65(3):467–471. https://doi.org/10.1007/s00280-009-1051-4
Gupta E, Mick R, Ramirez J, Wang X, Lestingi TM, Vokes EE, Ratain MJ (1997) Pharmacokinetic and pharmacodynamic evaluation of the topoisomerase inhibitor irinotecan in cancer patients. J Clin Oncol 15(4):1502–1510. https://doi.org/10.1200/JCO.1997.15.4.1502
Toffoli G, Sharma MR, Marangon E, Posocco B, Gray E, Mai Q, Buonadonna A, Polite BN, Miolo G, Tabaro G, Innocenti F (2017) Genotype-guided dosing study of FOLFIRI plus bevacizumab in patients with metastatic colorectal cancer. Clin Cancer Res 23(4):918–924. https://doi.org/10.1158/1078-0432.CCR-16-1012
Innocenti F, Schilsky RL, Ramirez J, Janisch L, Undevia S, House LK, Das S, Wu K, Turcich M, Marsh R, Karrison T, Maitland ML, Salgia R, Ratain MJ (2014) Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J Clin Oncol 32(22):2328–2334. https://doi.org/10.1200/JCO.2014.55.2307
van der Bol JM, Mathijssen RH, Creemers GJ, Planting AS, Loos WJ, Wiemer EA, Friberg LE, Verweij J, Sparreboom A, de Jong FA (2010) A CYP3A4 phenotype-based dosing algorithm for individualized treatment of irinotecan. Clin Cancer Res 16(2):736–742. https://doi.org/10.1158/1078-0432.CCR-09-1526
Denlinger CS, Blanchard R, Xu L, Bernaards C, Litwin S, Spittle C, Berg DJ, McLaughlin S, Redlinger M, Dorr A, Hambleton J, Holden S, Kearns A, Kenkare-Mitra S, Lum B, Meropol NJ, O’Dwyer PJ (2009) Pharmacokinetic analysis of irinotecan plus bevacizumab in patients with advanced solid tumors. Cancer Chemother Pharmacol 65(1):97–105. https://doi.org/10.1007/s00280-009-1008-7
Chabot GG (1997) Clinical pharmacokinetics of irinotecan. Clin Pharmacokinet 33(4):245–259. https://doi.org/10.2165/00003088-199733040-00001
Conti JA, Kemeny NE, Saltz LB, Huang Y, Tong WP, Chou TC, Sun M, Pulliam S, Gonzalez C (1996) Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol 14(3):709–715. https://doi.org/10.1200/JCO.1996.14.3.709
Sissung TM, Rajan A, Blumenthal GM, Liewehr DJ, Steinberg SM, Berman A, Giaccone G, Figg WD (2019) Reproducibility of pharmacogenetics findings for paclitaxel in a heterogeneous population of patients with lung cancer. PLoS One 14(2):e0212097. https://doi.org/10.1371/journal.pone.0212097
Ravegnini G, Urbini M, Simeon V, Genovese C, Astolfi A, Nannini M, Gatto L, Saponara M, Ianni M, Indio V, Brandi G, Trino S, Hrelia P, Biasco G, Angelini S, Pantaleo MA (2018) An exploratory study by DMET array identifies a germline signature associated with imatinib response in gastrointestinal stromal tumor. Pharmacogenomics J. https://doi.org/10.1038/s41397-018-0050-4
Uchiyama T, Kanno H, Ishitani K, Fujii H, Ohta H, Matsui H, Kamatani N, Saito K (2012) An SNP in CYP39A1 is associated with severe neutropenia induced by docetaxel. Cancer Chemother Pharmacol 69(6):1617–1624. https://doi.org/10.1007/s00280-012-1872-4
Nieuweboer AJ, Smid M, de Graan AM, Elbouazzaoui S, de Bruijn P, Eskens FA, Hamberg P, Martens JW, Sparreboom A, de Wit R, van Schaik RH, Mathijssen RH (2016) Role of genetic variation in docetaxel-induced neutropenia and pharmacokinetics. Pharmacogenomics J 16(6):519–524. https://doi.org/10.1038/tpj.2015.66
Rumiato E, Boldrin E, Amadori A, Saggioro D (2013) DMET (drug-metabolizing enzymes and transporters) microarray analysis of colorectal cancer patients with severe 5-fluorouracil-induced toxicity. Cancer Chemother Pharmacol 72(2):483–488. https://doi.org/10.1007/s00280-013-2210-1
Harris M, Bhuvaneshwar K, Natarajan T, Sheahan L, Wang D, Tadesse MG, Shoulson I, Filice R, Steadman K, Pishvaian MJ, Madhavan S, Deeken J (2014) Pharmacogenomic characterization of gemcitabine response—a framework for data integration to enable personalized medicine. Pharmacogenet Genomics 24(2):81–93. https://doi.org/10.1097/FPC.0000000000000015
Thompson P, Wheeler HE, Delaney SM, Lorier R, Broeckel U, Devidas M, Reaman GH, Scorsone K, Sung L, Dolan ME, Berg SL (2014) Pharmacokinetics and pharmacogenomics of daunorubicin in children: a report from the Children’s Oncology Group. Cancer Chemother Pharmacol 74(4):831–838. https://doi.org/10.1007/s00280-014-2535-4
Mathijssen RH, Marsh S, Karlsson MO, Xie R, Baker SD, Verweij J, Sparreboom A, McLeod HL (2003) Irinotecan pathway genotype analysis to predict pharmacokinetics. Clin Cancer Res 9(9):3246–3253
Li M, Seiser EL, Baldwin RM, Ramirez J, Ratain MJ, Innocenti F, Kroetz DL (2018) ABC transporter polymorphisms are associated with irinotecan pharmacokinetics and neutropenia. Pharmacogenomics J 18(1):35–42. https://doi.org/10.1038/tpj.2016.75
Wong M, Balleine RL, Blair EY, McLachlan AJ, Ackland SP, Garg MB, Evans S, Farlow D, Collins M, Rivory LP, Hoskins JM, Mann GJ, Clarke CL, Gurney H (2006) Predictors of vinorelbine pharmacokinetics and pharmacodynamics in patients with cancer. J Clin Oncol 24(16):2448–2455. https://doi.org/10.1200/JCO.2005.02.1295
Di Martino MT, Arbitrio M, Leone E, Guzzi PH, Rotundo MS, Ciliberto D, Tomaino V, Fabiani F, Talarico D, Sperlongano P, Doldo P, Cannataro M, Caraglia M, Tassone P, Tagliaferri P (2011) Single nucleotide polymorphisms of ABCC5 and ABCG1 transporter genes correlate to irinotecan-associated gastrointestinal toxicity in colorectal cancer patients: a DMET microarray profiling study. Cancer Biol Ther 12(9):780–787. https://doi.org/10.4161/cbt.12.9.17781
Hoskins JM, Marcuello E, Altes A, Marsh S, Maxwell T, Van Booven DJ, Pare L, Culverhouse R, McLeod HL, Baiget M (2008) Irinotecan pharmacogenetics: influence of pharmacodynamic genes. Clin Cancer Res 14(6):1788–1796. https://doi.org/10.1158/1078-0432.CCR-07-1472
Glimelius B, Garmo H, Berglund A, Fredriksson LA, Berglund M, Kohnke H, Bystrom P, Sorbye H, Wadelius M (2011) Prediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancer. Pharmacogenomics J 11(1):61–71. https://doi.org/10.1038/tpj.2010.10
Marcuello E, Altes A, Menoyo A, Rio ED, Baiget M (2006) Methylenetetrahydrofolate reductase gene polymorphisms: genomic predictors of clinical response to fluoropyrimidine-based chemotherapy? Cancer Chemother Pharmacol 57(6):835–840. https://doi.org/10.1007/s00280-005-0089-1
Li P, Chen Q, Wang YD, Ha MW (2014) Effects of MTHFR genetic polymorphisms on toxicity and clinical response of irinotecan-based chemotherapy in patients with colorectal cancer. Genet Test Mol Biomarkers 18(5):313–322. https://doi.org/10.1089/gtmb.2013.0494
Dias MM, McKinnon RA, Sorich MJ (2012) Impact of the UGT1A1*28 allele on response to irinotecan: a systematic review and meta-analysis. Pharmacogenomics 13(8):889–899. https://doi.org/10.2217/pgs.12.68
Dias MM, Pignon JP, Karapetis CS, Boige V, Glimelius B, Kweekel DM, Lara PN, Laurent-Puig P, Martinez-Balibrea E, Paez D, Punt CJ, Redman MW, Toffoli G, Wadelius M, McKinnon RA, Sorich MJ (2014) The effect of the UGT1A1*28 allele on survival after irinotecan-based chemotherapy: a collaborative meta-analysis. Pharmacogenomics J 14(5):424–431. https://doi.org/10.1038/tpj.2014.16
Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL (2007) UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 99(17):1290–1295. https://doi.org/10.1093/jnci/djm115
Liu D, Li J, Gao J, Li Y, Yang R, Shen L (2017) Examination of multiple UGT1A and DPYD polymorphisms has limited ability to predict the toxicity and efficacy of metastatic colorectal cancer treated with irinotecan-based chemotherapy: a retrospective analysis. BMC Cancer 17(1):437. https://doi.org/10.1186/s12885-017-3406-2
Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramirez J, Relling M, Chen P, Das S, Rosner GL, Ratain MJ (2009) Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol 27(16):2604–2614. https://doi.org/10.1200/JCO.2008.20.6300
Chen S, Villeneuve L, Jonker D, Couture F, Laverdiere I, Cecchin E, Innocenti F, Toffoli G, Levesque E, Guillemette C (2015) ABCC5 and ABCG1 polymorphisms predict irinotecan-induced severe toxicity in metastatic colorectal cancer patients. Pharmacogenet Genomics 25(12):573–583. https://doi.org/10.1097/FPC.0000000000000168
Hahn RZ, Antunes MV, Verza SG, Perassolo MS, Suyenaga ES, Schwartsmann G, Linden R (2019) Pharmacokinetic and pharmacogenetic markers of irinotecan toxicity. Curr Med Chem 26(12):2085–2107. https://doi.org/10.2174/0929867325666180622141101
Hahn RZ, Arnhold PC, Andriguetti NB, Schneider A, Kluck HM, Dos Reis SL, Bastiani MF, Kael I, da Silva ACC, Schwartsmann G, Antunes MV, Linden R (2018) Determination of irinotecan and its metabolite SN-38 in dried blood spots using high-performance liquid-chromatography with fluorescence detection. J Pharm Biomed Anal 150:51–58. https://doi.org/10.1016/j.jpba.2017.11.079
Acknowledgements
Funded by the Australian National Health and Medical Research Council Grant 628564.
Author information
Authors and Affiliations
Contributions
Wrote Manuscript, M Michael, W Liauw, S-A McLachlan, E Link, A Matera, M Thompson, M Jefford, RJ Hicks, C Cullinane, A Hatzimihalis, IG Campbell, S Rowley, PJ Beale, CS Karapetis, T Price, ME. Burge. Designed Research, M Michael, E Link, A Matera, M Thompson, RJ Hicks, C Cullinane, A Hatzimihalis, IG Campbell, S Rowley, Performed Research, M Michael, W Liauw, S-A McLachlan, E Link, A Matera, M Thompson, M Jefford, RJ Hicks, C Cullinane, A Hatzimihalis, IG Campbell, S Rowley, PJ Beale, CS Karapetis, T Price, ME. Burge. Analysed Data, and Contributed Analytical Tools. M Michael, E Link, M Thompson, RJ Hicks, C Cullinane, A Hatzimihalis, IG Campbell, S Rowley.
Corresponding author
Ethics declarations
Conflicts of interest
All contributing authors have no competing financial interests in relation to the work described.
Ethics approval
The study was approved by the Peter MacCallum Cancer Centre Ethics Committee on the 3rd Sept 2010, followed shortly after the Institutional Ethics Committees at all other study sites. This study was to be carried out in compliance with the protocol and with adherence to Good Clinical Practice, as described in the following documents: ICH Harmonized Tripartite Guidelines for Good Clinical Practice 1996. Directive 91/507/EEC, Rules Governing Medicinal Products in the European Community. Declaration of Helsinki, concerning medical research in humans (Recommendations Guiding Physicians in Biomedical Research Involving Human Patients, Helsinki 1964, amended Tokyo 1975, Venice 1983, Hong Kong 1989, Somerset West 1996, Edinburgh 2000, Washington 2002; Appendix 11).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Michael, M., Liauw, W., McLachlan, SA. et al. Pharmacogenomics and functional imaging to predict irinotecan pharmacokinetics and pharmacodynamics: the predict IR study. Cancer Chemother Pharmacol 88, 39–52 (2021). https://doi.org/10.1007/s00280-021-04264-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04264-8