Abstract
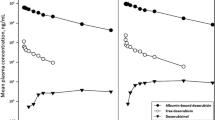
The purpose of the present study was to investigate the pharmacokinetics and pharmacodynamics of the new morpholino anthracycline drug MX2. A total of 27 patients with advanced cancer participated in a dose-escalation study in the first cycle of treatment with drug given i.v. at doses of 10–50 mg/m2 (total dose 16.8–107.5 mg). The mean total systemic plasma clearance (CL) of MX2 was 2.98 ± 1.68 l/min, the mean volume of distribution at steady state was 1460 ± 749 l and mean elimination half-life was 10.8 ± 5.1 h. The area under the plasma concentration-time curve (AUC) of MX2 was linearly related to the dose per kilogram and the dose per body surface area (r 2 = 0.43, P < 0.01 and r 2 = 0.44, P < 0.01, respectively). CL did not correlate with total body weight, lean body mass or body surface area. The mean elimination half-lives of the metabolites M1, M2, M3 and M4 were 11.8 ± 5.0, 21.9 ± 11.8, 19.0 ± 11.3 and 12.3 ± 6.3 h, respectively. The fractional E max model produced a much better fit to the relative nadir neutrophil count versus dose data (r 2 = 0.42) than to the relative nadir neutrophil count versus AUC or peak concentration (C max) data (r 2 = 0.15 and 0.09, respectively). There seemed to be a threshold dose of about 65 mg of MX2 at or above which a large proportion of patients had a nadir neutrophil count of less than 0.5 × 109/l. This study shows that the pharmacokinetics of MX2 are similar to those of other anthracyclines. With other anthracyclines the degree of myelosuppression seems to depend more on the AUC and C max than on the delivered dose; however, with MX2 the degree of myelosuppression depends more on the dose given than on drug exposure expressed as the AUC or C max.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 18 February 1996 / Accepted: 20 December 1996
Rights and permissions
About this article
Cite this article
Morgan, D., Hill, J., Clarke, K. et al. Pharmacokinetics and pharmacodynamics of MX2 hydrochloride in patients with advanced malignant disease. Cancer Chemother Pharmacol 40, 202–208 (1997). https://doi.org/10.1007/s002800050647
Issue Date:
DOI: https://doi.org/10.1007/s002800050647