Abstract
Tumour microenvironment is a complex ecosystem in which myeloid cells are the most abundant immune elements. This cell compartment is composed by different cell types, including neutrophils, macrophages, dendritic cells, and monocytes but also unexpected cell populations with immunosuppressive and pro-tumour roles. Indeed, the release of tumour-derived factors influences physiological haematopoiesis producing unconventional cells with immunosuppressive and tolerogenic functions such as myeloid-derived suppressor cells. These pro-tumour myeloid cell populations not only support immune escape directly but also assist tumour invasion trough non-immunological activities. It is therefore not surprising that these cell subsets considerably impact in tumour progression and cancer therapy resistance, including immunotherapy, and are being investigated as potential targets for developing a new era of cancer therapy. In this review, we discuss emerging strategies able to modulate the functional activity of these tumour-supporting myeloid cells subverting their accumulation, recruitment, survival, and functions. These innovative approaches will help develop innovative, or improve existing, cancer treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advanced understanding of immune system regulation circuits and biological insights of tumour-immune system interplay completely revolutionised the concept of cancer therapy. An example of this is the paramount success of immune checkpoint therapy (ICT) based on antibody-dependent targeting of T cell functional modulators like cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) that relies on cancer restriction through the activation of the host immune system and resulted in significant improved clinical benefits in many type of solid tumours [1,2,3]. However, ICT does not work yet as single agent in patients affected by tumours with specific histology and genetic features (e.g. in pancreatic cancer and glioblastoma) [4]. Moreover, even in tumour characterised by high mutation burden, such as melanoma and breast cancer, ICT improved the clinical outcome only in a small fraction of treated patients [1]. Numerous factors regulate the dynamic process of tumour immunity and response to immune checkpoint blockade that can be broadly categorised in two main classes: tumour-intrinsic and tumour-extrinsic factors [2, 5].
Over the tumour evolution, cancer cells acquire several mutations leading the expression of proteins with an altered folding or mutated encoding gene sequences that allow to generate new immunogenic peptides able to activate a specific immune reaction. These neo-antigens are able to evoke a potential tumour-restricted response since they are distinct from self-antigens [6]. Conversely, genetic and epigenetic modifications leading alterations of both antigen presentation machinery [7] and signal transduction pathways, such as interferon (IFN)γ-signalling defects [8], negatively influence the responsiveness of ICT. Tumour-extrinsic factors of immune resistance are dependent on tumour-microenvironment (TME) components. Indeed tumour can be considered a complex tissue in which neoplastic cells, immune cells, vascular components (e.g. endothelial cells), fibroblasts, and matrix interplay defines the fate of ICT. For instance, the CD8+ T cell localisation at tumour margins and within the tumour prior to ICT correlated positively with a robust response to immunotherapy [9], whilst regulatory T lymphocytes (Tregs) blunting the effector immune functions contributed to the clinical failure of ICT [10]. However, the most pervasive mechanism activated by tumours to alter the immune response in TME is the induction of an emergency haematopoiesis pushing the accumulation of myeloid cells with immunosuppressive functions and pro-inflammatory properties, such as myeloid-derived suppressor cells (MDSCs) [11, 12]. Indeed, these tumour-reprogrammed myeloid cells have the ability to support tumour progression by assisting tumour cell survival, angiogenesis, and metastatic process [13,14,15]. Collectively, tumour cells hijack both innate and adaptive immune resistance mechanisms to avoid immune-based clearance [16]. Accordingly, immune suppression, inflammation, abnormal differentiation, and function of myeloid cells are enlisted as hallmarks of cancer [17].
Here we review evidences indicating that targeting tumour-reprogrammed myeloid cells may be beneficial in promoting response to ICT. To develop more effective myeloid cell-targeted therapies is indeed mandatory to combine data of single-cell transcriptome, metabolic, and epigenetic profiles to pinpoint complex relationships between myeloid cells and other TME components. We think that immunotherapy should be considered an immune system rather than a cancer treatment and, therefore, any improvement will depend on overcoming the gaps in understanding the biology of TME immune components with particular focus on myeloid cells.
Myeloid cells in tumour microenvironment
Immune cell heterogeneity entangles TME immune profiling [18]. In this context, myeloid cells represent the most abundant and functionally plastic immune components promoting both tumour recognition and escape. Indeed, myeloid cells rapidly infiltrate early neoplastic lesions and may dictate the fate of tumours by supporting either T cell-mediated killing by acting as professional tumour antigen-presenting cells or promoting immune arrest and cancer progression by inhibiting both adaptive and innate immunity. Tumour-associated macrophages (TAMs), MDSCs, tumour-associated neutrophils (TANs), and dendritic cells (DCs) are major tumour-infiltrating myeloid cells (TIMs) [19]. Myeloid cells identified in tumours have different ontogeny (i.e. bone marrow [BM]-derived or tissue resident cells) and are characterised by a plastic phenotype that can be shaped by cytokines and other soluble factors. Tumours support the emergency myelopoiesis favouring the generation of unconventional mature and immature myeloid cells endowed with tumour-promoting activities [20, 21]. For instance, a significant portion of tumour-infiltrating myeloid cells are of erythroid origin [22], highlighting how tumour reprograms myeloid cell differentiation. In this scenario, TAMs and MDSCs represent the ultimate commitment of the tumour-dependent myeloid-cell reprogramming [23, 24] and will be the focus of this review. For more detailed information on other tumour-infiltrating myeloid cells, please refer to the following manuscripts [25,26,27,28,29].
TAMs can be polarised towards an inflammatory (M1-like) or anti-inflammatory (M2-like) phenotype which supports immune control or immune evasion of neoplastic cells, respectively [30]. Notably, M1/M2 macrophage dichotomy is an oversimplification of the heterogeneity of macrophages that occurs in vivo [31]. TAMs can be polarised towards an inflammatory (M1-like) or anti-inflammatory (M2-like) phenotype which supports immune control or immune evasion of neoplastic cells, respectively. Notably, M1/M2 macrophage dichotomy is an oversimplification of the heterogeneity of macrophages that occurs in vivo. Indeed, M1- and M2-polarised macrophages should be viewed as the extremes of macrophage plasticity since TAM with identifiable M1 or M2 polarisation do not really exist in the tumours, instead being represented by TAM with mixed characteristics. However, it is interesting that this M1/M2 macrophage classification may explain the correlation between TME-infiltrating TAMs and patient outcome [32]. M1-TAMs are characterised by sustained phagocytosis and enhanced anti-tumour inflammatory reactions. This cell subset supports T cell activation by expressing co-stimulatory molecules (i.e. CD80, CD86) and high levels of major histocompatibility complex class II (MHCII) molecules. Instead, alternatively activated M2-TAMs support tumour cell survival, angiogenesis, and invasion [21]. These macrophages are characterised by higher expression of CD163 and CD206 both in humans and mice [33]. M2-TAMs can promote tumour progression by both soluble mediators (e.g. Arginase [Arg]1-derived products and transforming growth factor beta [TGFβ] release) and surface receptors (e.g. expression of programmed death-ligand 1 [PD-L1]), resulting in suppression of anti-tumour response [21]. Moreover, these cells actively support metastatic process by remodelling the extracellular matrix. Indeed, we recently showed that disabled homolog 2 mitogen-responsive phosphoprotein (DAB2)-expressing M2-TAMs play central role in lung metastasis formation by remodelling tissue matrix components [34]. Clinically, patients with tumour enriched in macrophages, especially the ones with pro-angiogenic features, have a poor prognosis and reduced overall survival [26, 31, 35,36,37]. TAMs were also reported to mediate chemotherapy resistance in different cancer settings by activating anti-apoptotic pathways [38]. Furthermore, TAMs play a negative role in ICT such as by preventing cytotoxic T cells from reaching tumour cells [39] as well as by promoting antibody clearance activity through Fc-Fcγ receptor binding [40]. For all these reasons, emerging macrophage-related therapeutic approaches aiming to deplete and/or shift macrophage polarisation are now promising therapeutic strategies for cancer patients.
MDSCs, a heterogeneous population of inflammation-biased monocytic (M-MDSCs) and polymorphonuclear (PMN-MDSCs) cells with immune suppressive features, share with TAMs several pro-tumour functions [23]. Whether MDSCs have a different ontogeny or represent an alternative polarisation/differentiation status of monocytes and neutrophils is still debated, yet, M- and PMN- MDSCs share many surface antigens with monocytes and neutrophils, respectively. Nonetheless, some specific markers have been recently recognised. For example, human PMN-MDSCs expressing lectin-type oxidised LDL receptor 1 (Lox1) are encountered in both blood and tumours of cancer patients and their presence is associated with worse clinical outcome [41, 42]. Moreover, CD84 protein has been identified as a specific PMN-MDSC marker in both genetically engineered mouse models (GEMM) and breast cancer patients [43]. Indeed, many investigators reported the accumulation in the PBMC fraction of low-density neutrophils (LDN) expressing CD15 and CD66b (markers shared between PMNs and PMN-MDSCs) and endowed with immune suppressive features, reminiscent of PMN-MDSCs, in individuals affected by different diseases, as cancer [44], bacterial sepsis [45], and COVID-19 [46, 47]. Despite being associated with diseases, PMN-MDSCs can also increase under physiological conditions (e.g. pregnancy [48]) or pharmacological treatments [49]. We demonstrated that both the expansion and the immunosuppressive function of MDSCs are abrogated in the absence of CCAAT/enhancer binding protein (c/EBP) β, in tumour-bearing mice, confirming the crucial role of this transcriptional factor in tumour-reprogrammed myeloid cells [50]. Notably, macrophages that are differentiated from M-MDSCs, but not from monocytes, are immune suppressive showing a restricted M-MDSC-associated genomic profile and characterised by the persistent expression of S100A9 [51]. Finally, immunosuppressive functions of MDSCs and TAMs in TME are also enforced by environmental metabolic switches such as nonderepressible-2 kinase (GCN2), confirming the intrinsic plasticity of these myeloid cell subsets [52]. In humans, M-MDSCs can be distinguished from monocytes based on low expression levels of HLA-DR [53] and activation of signal transducer and activator of transcription 3 (STAT3)-dependent signalling pathway [54, 55]. M-MDSC generation, differentiation, and function are strictly controlled by several signalling pathways which are controlled by key transcriptional factors as c/EBPβ, STAT3, and nuclear factor kappa-light chain enhancer of activated B cell (NF-κB). Recently, the compromised translocation of NF-κB p50 protein was reported to arrest the release of protein acidic and rich in cysteine (SPARC), thus abrogating reactive oxygen species (ROS)-dependent MDSC-associated immunosuppression. Indeed, the blockade of p50 translocation into the nucleus impairs the generation of immunosuppressive p50:p50 homodimers in favour of the p65:p50 inflammatory heterodimers [56]. The critical role of NF-κB p50 protein in driving MDSC differentiation has been also confirmed by the nuclear translocation of a protein complex formed by p50 in association with cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory proteins (c-FLIP) [57]. Indeed, c-FLIP in tumour-reprogrammed monocytes does not only act as anti-apoptotic protein but also drives a marked regulation of genes encoding for immunosuppression-associated factors, like PD-L1, PD-L2, and IL-10 [57, 58]. On the other hand, aberrant FLIP expression in monocytes orchestrates pro-inflammatory pathways, leading to an unusual cytokine production fuelling massive cytokine release, feature of the cytokine release syndrome (CRS) [59]. Conversely, FLIP genetic deletion completely abrogates the generation of M-MDSCs [60], highlighting FLIP as a key regulator of this cell subset. These findings point to FLIP as a key functional-fate determinant of MDSCs, and as a novel targeting candidate to control cancer-associated immune dysfunctions.
Last-generation technologies (such as sc-RNA-seq and CYTOF) dramatically improved our current understanding of myeloid cell ontogeny and functional polarisation in cancer [61, 62] and other pathologies. To solve the myeloid-cell puzzle, Sanin and colleagues established a predictive macrophage activation model across 12 tissues and 25 biological conditions, in mice, and identified shared and unique functional pathways [63]. Moreover, TME deconvolution of about 210 patients across 15 human cancers identified many different subsets of TAMs with mixed M1/M2 signatures [64]. By taking advantage of pro-inflammatory and pro-angiogenic molecular signatures to address functional properties of different TAM subsets, the authors identified interferon-stimulated gene 15 (ISG15) and secreted phosphoprotein 1 (SPP1) gene signatures as TAMs prototypes of anti-tumour and pro-tumour macrophages, respectively. Notably, (SPP1)+/− cells synergise with tumour-specific fibroblast activation protein (FAP)+ fibroblasts to establish a desmoplastic reaction, which limits the infiltration of cytotoxic T cells, thus restricting efficacy of checkpoint inhibitor therapy [65]. A similar macrophage’s heterogeneity has been identified in mouse models of colorectal cancer. In this context, the antibody-dependent blockade of the colony-stimulating factor-1 receptor (CSF1R) depleted TAMs with an inflammatory signature, sparing the pro-angiogenic ones [66], suggesting the use of alternative therapeutic approaches that re-educate in spite of deplete TAMs [67]. Taking together, newly available high-throughput datasets can be interrogated to expand the understanding of myeloid cell biology and define their contribution to tumour progression. Implementation of single-cell omic technology to both GEMMs and human specimens represent the new avenue to evaluate new approaches re-educating TIMs towards an anti-tumour role and for predicting patient response to therapy.
Targeting tumour-reprogrammed myeloid cells
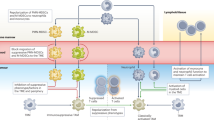
Compelling evidence of immune system role in tumour evolution revolutionised the development of therapies supporting immune system education to achieve a long-term control of tumour and eventually its complete debulking, other than targeting directly tumour cells. Improving immunological performances against cancer can be achieved by targeting immune-checkpoint inhibitors on adaptive immune cells for sustaining their survival and functions but also selective modulators on innate cells able to support efficiently T cell activation and limit their immunosuppressive functions. Developing effective myeloid cell-targeted approaches is a challenging research field since myeloid cells are elusive elements able to modulate their metabolism, cell-surface marker expression, and release a variety of soluble factors; therefore, the most effective strategy must aim to efficiently modulating myeloid cells’ plastic nature. Here we summarise recent findings of key players orchestrating myeloid cell biology which can be exploited to turn foes in friends (Fig. 1).
Strategies for myeloid cell reprogramming towards an anti-tumor phenotype. Myeloid cells are reprogrammed at epigenetic, transcriptional, and functional levels by tumor cells to support cancer outgrowth. Use of specific inhibitors (red) can deplete them, or avoid their recruitment in tumor. Alternatively, blocking immune suppressive switches (red Ͱ) and activating (green arrow) pro-inflammatory sensors can re-educate myeloid cells to support anti-tumor immunity. ARG1—arginase 1, ATP—adenosine triphosphate, c-FLIP—cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein, cGAS—cGAMP synthase, CLEVER—common lymphatic endothelial and vascular endothelial receptor, CCR2—C–C motif chemokine receptor 2, cEBP-β—CCAAT/enhancer-binding protein beta, CSF1R—colony-stimulating factor 1 receptor, DNMT3A—DNA cytosine-5-methyltransferase 3, EZH2—enhancer of zeste homolog 2, GPCR—G protein-coupled receptors, HIF—hypoxia-inducible factor, HDAC—histone deacetylase, IDO1—indoleamine 2,3-dioxygenase 1, IL—interleukin, JAK—Janus kinase, MARCO—macrophage receptor, mTOR—mammalian target of rapamycin, NF-κB—nuclear factor kappa-light chain enhancer of activated B cells, NOS2—nitric oxide synthase 2, NOX2—NADPH oxidase, PI3K—phosphoinositide 3-kinases, RNS—reactive nitrogen species, ROS—reactive oxygen species, PDL1—programmed death-ligand 1, SIGLEC—the sialic acid-binding immunoglobulin-like lectin, STAT—signal transducers and activator of transcription, STING—stimulator of interferon genes, TGF—transforming growth factor, TLR – toll-like receptor, TNF—tumor necrosis factor, TREM—triggering receptor expressed on myeloid cells, TYK—tyrosine protein kinase, VEGF—Vascular endothelial growth factor
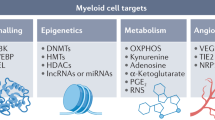
Targeting MDSC-dependent signalling pathways
Signalling and chronic-tumour dependent hematopoietic stimulation through the release of CSF-2, CSF-3, IL-6, and other cytokines are associated with the activation of STAT3 signalling and expression of c/EBPβ transcription factor [68] which sustain MDSC expansion and immune suppressive features [50]. In accordance, c-Rel-dependent c/EBPβ upregulation orchestrates the generation of MDSCs with potent pro-tumour features [69]. Akt/mTOR-dependent activation of phosphoinositol 3 kinase pathway (PI3Kγ) triggers through the expression of c/EBPβ an immunosuppressive transcriptional program in myeloid cells supporting tumour progression [70]. Tumour-biased monocytes upregulate c-FLIP, which in turn activates an immune suppressive program, partially by NF-κB activation, including IL-10, IDO-1, and PD-L1 expression [57]. Thus, targeting of either STAT3, or c-Rel, or PI3Kγ reprogram myeloid cells towards an anti-tumour phenotype resulting in the sculpting of a TME towards the support of cytotoxic T cell response and restricting tumour progression in preclinical models of solid cancers [69,70,71,72,73]. Notably, some of these targeting strategies will enter soon the clinical phase, alone, or in combination with immunotherapy (Table 1). Given the antithetical role of c/EBPα (pro-inflammatory) and c/EBPβ (anti-inflammatory), and preclinical data supporting the hypothesis of c/EBPα triggering could empower anti-tumour immunity, MTL-CEBPA (an RNA-based agonist of c/EBPα) has been recently proposed in combination with anti-PD1 for the treatments of solid tumours (Table 1). However, given the broad cellular expression of several pathways and their role for physiologic regulation of tissue homeostasis and body functions, on-target and pathway-related side effects must be taken into account. c/EBP homologous protein (CHOP), which is an apoptosis-related transcription factor induced by endoplasmic reticulum (ER) stress, is essential for MDSC’s immune regulatory function [74]. CHOP expression in MDSCs was induced by tumour-linked ROS and RNS and it was regulated by the activating-transcription factor-4 (ATF4). CHOP-deficient MDSCs displayed reduced signalling through c/EBPβ, leading to a decreased production of IL-6 and low expression of phospho-STAT3 [74]. Therefore, these data highlight Chop/c/EBPβ axes as main driver of tumour-induced tolerance and targeting CHOP might represent a new valuable way to improve current cancer immunotherapies. STAT3 phosphorylated by the Janus-activated kinase (JAK) family is considered a hallmark of MDSCs [14]. STAT3 prevents MDSC apoptosis and promotes their expansion by mediating the expression of apoptosis inhibitors, including Bcl-XL, cyclin D, and cMyc. In addition, activation of STAT3 drives the production of the calcium-binding inflammatory protein, S100A8/9, and increases the accumulation of MDSCs by limiting DC differentiation and expansion [75]. ARG1 is also a downstream target of STAT3 in circulating and infiltrating MDSCs. Similarly, NADPH oxidase 2 (NOX2), induced by STAT3 in MDSCs, generates ROS that can prevent DC differentiation and antigen presentation. Finally, STAT3 blocks myeloid cell differentiation by downregulating the expression of IRF8, a transcription factor driving the development of monocytes and DCs whilst limiting granulocyte development [76]. As shown by genetic studies in mice, IRF8 inhibition is indeed responsible for halting of the PMN-MDSC differentiation and expansion [77]. Conversely, a decrease in STAT3 signalling can enable MDSC differentiation into TAMs, which often becomes the dominant tumour-infiltrating myeloid cell population [78]. Therefore, targeting STAT3 is an attractive strategy to alleviate MDSC-mediated immunosuppression in the tumour microenvironment without the need for myeloid cell depletion.
Targeting epigenetic modifications
DNA methylation and histone acetylation/deacetylation catalysed, respectively, by DNA methyl-transferases (DNMTs) and histone acetyltransferases (HATs) and deacetylases (HDACs) are often altered in tumour-reprogrammed myeloid cells. Indeed, many epigenetic mechanisms influence MDSC differentiation and functions, thus remodelling TME [79]. Hence, a pharmacological targeting of epigenetic modifiers can be potentially considered for combinatorial immunotherapy approaches aiming at both triggering an immuno-mediated tumour cell recognition and, simultaneously, reducing MDSC-dependent immune suppression. EZH2 inhibition, a histone-lysine N-methyltransferase, has reported to suppress anti-tumour immunity by driving MDSC differentiation from primitive hematopoietic progenitors reducing CD4+ and IFNγ+CD8+ T cells in lung and colon carcinoma models [80]. On the other hand, a pan-HDAC inhibition decreased MDSC accumulation in a mammary tumour model by promoting the apoptosis of MDSC-differentiating Gr-1+ cells. This selective depletion was partially due to increased intra-cellular reactive oxygen species that, consequently, was associated to an increased proportion of IFNγ- or perforin-producing CD8+ T cells [81]. Recently, histone (de)acetylation has shown to be involved in the polarisation of M-MDSCs in PMN-MDSCs [82]. In detail, Youn and colleagues have reported that a large proportion of M-MDSCs in tumour-bearing mice could acquire phenotypic, morphological, and functional features of PMN-MDSCs, rather than DCs or macrophages. This process is governed by epigenetic transcriptional silencing of the retinoblastoma gene controlled by HDAC-2. HDAC inhibition re-directs M-MDSC differentiation towards macrophages and DCs [82]. Furthermore, HDAC11 has emerged as a negative regulator of MDSC expansion and function in a lymphoma model by controlling IL-10 gene expression [83]. Indeed, immature myeloid cells to MDSC transition require a decreased expression of HDAC11 [83]. Conversely, the bromodomain of the HAT CBP/EP300 is a critical regulator of H3K27 acetylation in MDSC promoters and enhancers of pro-tumourigenic genes [84]. CBP/EP300-BRD inhibition redirects tumour-associated MDSCs from a suppressive to an inflammatory phenotype through STAT pathway-related gene downregulation and ARG1 and iNOS inhibition, thus limiting tumour growth in a model of colon carcinoma [84]. Interestingly, the epigenetic-associated component p66a, a subunit of the Mi2/NuRD HDAC complex, has shown to suppress STAT3 phosphorylation and ubiquitination by directly interacting with STAT3 protein, providing novel insights in controlling STAT3 activation in myeloid cell differentiation and activation [85]. On the other hand, class I HDAC inhibition has been reported to support anti-tumour response by dampening ARG-1, iNOS, and COX-2 levels in MDSCs as well as altering the release of cytokines/chemokines [86]. Interestingly, the inhibition of class I HDAC with entinostat selectively reduces the immunosuppressive activity mediated by PMN-MDSC, without any effect on M-MDSCs or macrophages [87]. Indeed, M-MDSC displayed higher levels of class II HDAC6, and its inhibition with ricolinostat reduces M-MDSC suppressive activity, without affecting PMN-MDSCs. However, only the combination of both molecules impacts on tumour progression [87].
MDSC properties are also influenced by specific changes in DNA methylation patterns. For instance, Durkin and colleagues demonstrated for the first time the ability of the demethylating agent decitabine to promote the differentiation of tumour-infiltrated CD11b+ cells into mature F4/80/CD11c/MHC class II-positive APCs [88]. Decitabine strongly reduces the release of immune suppressive and pro-inflammatory factors by tumour-derived myeloid cells, and, overall, naïve mice receiving ex vivo reprogrammed tumour-derived myeloid cells were protected from tumour outgrowth [88]. More recently, the downregulation of DNMT3A deletes MDSC-specific hypermethylation and abrogates their immunosuppressive capacity [89]. Ovarian cancer patient-derived MDSCs showed a similar hypermethylation signature in association with a prostaglandin E2 (PGE2)-dependent DNMT3A overexpression [89]. Furthermore, decitabine employment reduces tumour cell proliferation and trigger T cell immune response by depleting M-MDSCs in different tumour models [90, 91]. Taken together, these data suggest the control of DNA methylation as promising scenario for clinical approach (Table 1). On the other hand, the use of combinatorial epigenetic drugs not only could have an impact on tumour progression, but also might prevent the formation of the premetastatic niche [92]. In accordance, adjuvant epigenetic therapy with low-dose DNMT and HDAC inhibitors disrupts the premetastatic niche by blocking the trafficking of MDSCs through the downregulation of CCR2 and CXCR2 and also favouring MDSC differentiation into anti-tumour macrophage-like cells [92]. Collectively, these findings clearly reveal epigenetic drugs as potent tumour-reprogrammed myeloid cell-targeting agents that could be used to enhance the efficacy of ICT.
Targeting myeloid-cell recruitment networks
The recruitment of M-MDSCs is mainly mediated by tumour-expressing C–C motif chemokine ligand 2 (CCL2) in several tumour types, including breast, ovarian, gastric, and colorectal cancer [93]. Indeed, tumour-associated myeloid cells from patients frequently express CCR2 [94, 95]. Hence, blocking the CCL2-CCR2 interaction could be an effective therapeutic approach to prevent the accumulation of pro-tumour myeloid cells within TME. Promising results have been reported in several preclinical tumour models [96,97,98,99]. The seminal work by Pollard’s laboratory demonstrated that the therapeutic blockade of CCL2-CCR2 axis interrupts the recruitment of inflammatory monocytes and inhibits metastasis in vivo, prolonging the survival of mice bearing breast cancer [95]. However, the interruption of CCL2 inhibition has shown to promote a rebound of pro-tumour myeloid cells inducing mouse breast cancer metastasis [100]. Furthermore, prostate cancer patients enrolled in a phase II clinical trial using an anti-CCL2 monoclonal antibody showed an increased CCL2 release following anti-CCL2 interruption [101], suggesting that a continuous arrest of CCL2 is mandatory to control tumour progression. Several other cytokines and chemokine receptors have been reported to induce monocyte and neutrophil recruitment, like CCL5 and CCL7 [102, 103] and CCL3 [104]. Recently, a therapeutic approach by in vivo silencing of CCR1 and CCR5 on myeloid cells has shown to strongly inhibit tumour progression by converting PMN-MDSCs into anti-tumour neutrophils [105]. An interesting report showed that metastatic tumours often overexpressed CSF-3, leading to the expansion and mobilisation of Ly6G+Ly6C+ granulocytes, which in turn produced Bv8, a protein implicated in angiogenesis and mobilisation of myeloid cells. This process creates a positive feedback loop with the consequent accumulation of PMNs in organ-specific metastatic sites resulting in an increased metastatic ability [106]. Of note, recent studies suggest that CD200-CD200 receptor (CD200R) interaction might be essential in controlling the myeloid heterogeneity in tumours by a mechanism involving CD200-expressing endothelial cells [107, 108]. In addition, the expression of CD200 was found in human pancreatic cell lines and CD200R expression was found at high level on PDAC patient-derived MDSCs [108]. In vivo studies demonstrate that CD200 antibody blockade limits the percentage of tumour-infiltrating MDSCs, but the significance and the mechanisms underlying CD200-CD200R interaction in tumour microenvironment remain to be clarified [108].
Targeting MDSC-released cytokine and immune mediators
Secretion of soluble factors able to fuel a pathological inflammation or inhibit immunological responses in TME is considered a key mechanistic pathway of MDSC’s biology. Here we summarised the most relevant MDSC-released cytokines/growth factors able to sustain tumour progression.
Emergency myelopoiesis in BM is influenced by persistent stimulation from tumors. These signals include cytokines, namely, CSF-1, -2, and -3, whose function is often overlapped and converging to signaling pathways of the JAK/STAT/ERK/PI3K axes [112]. In this contest, it was shown that mouse breast 4T1 cancer cells release CSF-2 and CSF-1, promoting BM output of MDSCs and preparing the premetastatic niche [106]. Once in the lung, MDSCs induce angiogenesis and sustain the metastatic spread by, in turn, releasing CSF-2. Chemotherapy significantly enhanced the production of CSF-2 from various tumors (e.g. pancreatic adenocarcinoma, PDAC) [113]. Further, CSF-2 and CSF-3 were shown to induce the differentiation of monocytes into MDSCs and to stimulate myelopoiesis in BM of in mouse model of PDAC and glioblastoma [114].
The cytokine IL-6 was found to be a crucial regulator of MDSC accumulation, activation, and differentiation in vitro as well as a factor promoting tumour cell proliferation, survival, invasiveness, and metastasis. Accordingly, IL-6 can be used as a negative prognostic marker in cancer. Within the tumour microenvironment and in the periphery, IL-6 promotes differentiation of myeloid precursors into MDSCs and reinforces their suppressive function by promoting and maintaining STAT3 phosphorylation [115]. For example, phosphorylated STAT3 levels in MDSCs isolated from head and neck squamous cell carcinoma (HNSCC) patients positively correlate with ARG1 expression and suppression of autologous T cell proliferation [54]. Since STAT3 is a downstream regulator of IL-6, which was found to be associated with a worse survival in HNSCC patients [116], a correlation between IL-6 levels, STAT3 phosphorylation, and ARG1 expression could be proposed.
The cytokine IL-1β drives MDSC expansion and migration [117]. IL-1β concentration was positively correlated with M-MDSC subset [118] in the peripheral blood of advanced melanoma patients. Moreover, M-MDSC produces IL-1β, which in turn upregulates E-selectin expression, favouring tumour cell arrest on endothelial cells, in preclinical cancer models [119]. In other studies, tumour-derived NLRP3 increases the expression and secretion of IL-1β by MDSCs [120]. Currently, several agents are available to inhibit IL-1β, including IL-1Ra (anakinra), IL-1β-specific antibodies (canakinumab), and inflammasome inhibitors [121, 122]. Notably, multiple cancer therapeutic agents such as chemotherapeutic drugs, MAPK inhibitors, and BRAF V600E inhibitor (BRAFi) have been reported to either increase the expression of IL-1β or activate inflammasomes in myeloid cells causing unwanted side effects. In this regard, IL-1β blockade may generate adjunctive effects when combined with chemotherapies or other treatments in cancer.
TGF-β is a well-documented immunosuppressive cytokine secreted by MDSCs in tumour-bearing host [123]. How MDSC-derived TGF-β is released and regulated remains elusive. It was shown that MDSC-derived TGF-β is induced by IL-13 and CD1d-restricted T cells, most likely natural killer T (NKT) cells, in vivo [124]. Recent studies have shown that TGF-β production by MDSCs is regulated by TNF-α and semaphorin 4D, in vitro [125, 126]. In contrast, CD14+HLA-DR−/low MDSCs from patients with liver cancer show no TGF-β secretion [127], suggesting that TGF-β production by MDSCs may be context-dependent. MDSC-derived TGF-β contributes to T cell suppression. Song et al. have shown that tumour-derived MDSC transfer to asthmatic mice leads to reduced pulmonary recruitment of inflammatory cells, suppressed Th2 response, and decreased IgE production in a TGF-β1-dependent manner [128]. Furthermore, TGF-β is essential in Treg induction by MDSCs. In addition to immune suppression, TGF-β has been implicated in the regulation of tumour metastasis facilitated by MDSCs. A portion of tumour cells undergoes epithelial-mesenchymal transition (EMT) to disseminate, invade surrounding tissue, and metastasize. In a spontaneous murine model of melanoma, Toh and colleagues have shown for the first time that MDSCs use TGF-β, epidermal growth factor, and hepatocyte growth factor to induce EMT and to support metastasis and that MDSC depletion reverts this phenotype [125], confirming TGF-β as a critical target to abolish MDSC’s pro-tumour features.
Tumour-reprogrammed myeloid cells also actively influence angiogenesis, which represents a hallmark of cancer progression [17]. Anti-angiogenic therapies targeting vascular-endothelial growth factor (VEGF) and cognate receptors provided clinical benefits in different kind of solid tumours (including colorectal, kidney, lung, ovarian, brain tumours) [129], although often, response to therapy was limited in time and in a fraction of treated patients. In part this is mediated by compensatory circuits of sustained angiogenesis mediated by tumour-infiltrating myeloid cells [130,131,132]. In accordance, single-cell-mediated TIM deconvolution underscores the association of pro-angiogenic TAMs with worse overall survival in different solid tumour contexts [64]. Thus, repolarisation of TIMs from pro-angiogenic to anti-tumour phenotype can synergise with anti-angiogenic therapy to support long-term control of tumour progression. Clinical trials targeting VEGF and other soluble mediators are listed in Table 2.
Targeting metabolic pathways related to immune suppression
Myeloid cells can affect T cell recruitment and activity in TME by employing an arsenal of soluble mediators, including metabolites (i.e. NO, ROS, RNS, adenosine, α-ketoglutarate, prostaglandin, kynurenine, lactate) able to alter T cell function and survival [133, 134]. MDSCs sense and adapt to nutrient changes by acquiring the most effective pathways to maintain their immunosuppressive and pro-tumour functions. We detail below the most recent updates on major metabolic pathways.
Amino acid
Increased amino acid metabolism in MDSCs impairs anti-tumour T cell activity [135]. Especially, arginine, glutamine, and tryptophan have been shown to control viability, polarisation, and motility as well as effector function of anti-tumour T cells [42, 136]. Increased consumption of L-arginine by ARG1 is one of the known immunosuppressive mechanisms set in place by MDSCs to enhance tumour-immune escape. In fact, depletion of arginine down-modulates the expression of CD3ζ chain and alters GCN2 in T cells, resulting in TCR loss of function and cell cycle arrest, respectively [137,138,139]. Alongside, products derived from ARG1 metabolism, as ornithine, putrescine, and spermidine broadly promote MDSC immunosuppressive function, particularly in brain tumours [140, 141]. iNOS also contributes to fine tune the amount of L-arginine in the TME. The metabolic product derived from L-arginine degradation, NO, is known to increase T cell apoptosis by impairing IL-2R signalling trough JAK-3, STAT5, ERK, and Akt [142, 143]. In fact, NO directly increases DNA damage response, mitochondrial ROS generation, and produces peroxynitrites (PNT) in a NADPH-dependent manner, by reacting with superoxide anion [144, 145]. In addition, PNT favours post-translational modification of specific chemokines and cytokines (e.g. CCL2 and CSF-2) which have been shown to favour the recruitment and infiltration of anti-tumour T cells as well as they critically alter MHC-peptide complex inhibiting T cell activation [146,147,148]. Moreover, sustained type I interferon signalling exacerbate NO production by aberrant iNOS expression inducing anti-PD1 resistance [149]. On the other hand, NO produced by a subset of myeloid cells infiltrating the tumour, Tip-DCs, sustain the anti-tumour activity of adoptively transferred tumour-specific CD8+ T cells [150]. These data suggest that the outcome of amino acid depletion in the TME is cell dependent and future novel therapeutic strategies will have to consider.
Glutamine is one of the most abundant amino acids present in the blood. In cancer, glutamine is converted in glutamate and α-ketoglutarate, to support nucleoside and lipid biosynthesis, and to sustain protein glycosylation [151, 152]. Oncogenes, such as c-MYC and KRAS, greatly increase the uptake and catabolism of glutamine in cancer cells [153, 154], further enhancing the paucity of glutamine available in the TME for both tumour-promoting or -killing mechanisms. In MDSCs, L-glutamine fuels the tricarboxylic acid (TCA) cycle providing the intermediates and energy for the development and effector functions. Besides competing with tumour cells for glutamine, MDSCs oxidise L-glutamine in an AMPK-dependent manner, which increases and sustains their immunosuppression function [155]. In line with this observation, MDSCs were shown to increase glutamine biosynthesis and transglutaminase (TGM) activity in a murine model of metastatic mammary tumours [156] and TGM expression in MDSCs was correlated to the metastasis and multi-drug resistance of breast cancer [157].
Tryptophan catabolism is mediated in mammals by two closely related indoleamine-pyrrole 2,3 dioxygenase enzymes (indoleamine 2,3 dioxygenase [IDO]1 and IDO2) and the unrelated enzyme tryptophan 2,3 dioxygenase (TDO) [158]. IDO1 is primarily expressed by myeloid cells and stroma in response to inflammatory immune signals whereas IDO2 and TDO are largely unresponsive to immune stimuli and have a broader expression pattern [159]. Research examining the role of IDO1-mediated immune regulation has focused on the effect of amino acid consumption (i.e. amino acid starvation, stress) and the production of effector catabolites. IDO1-generated N-formyl-L-kynurenine is further catabolised by aryl formamidase to form L-kynurenine (L-Kyn). L-Kyn is a key product of IDO1 catabolism of tryptophan and, together with other downstream catabolic products (e.g. cinnabaric acid), is a regulator of immunity by direct binding to the aryl hydrocarbon receptor (AhR) [160]. AhR is a cytoplasmic receptor/transcription factor with a key role in immune function. AhR function in MDSCs has not been examined in detail. Nonetheless, AhR potently impacts hematopoietic progenitor development driving expansion of precursors [161] and AhR signalling may promote differentiation of leukemic stem cells in acute myeloid leukaemia. AhR signalling impacts MDSC expansion and differentiation by causing proliferation and differentiation of hematopoietic precursors and promoting the emergency granulopoiesis [50]. Together, these studies support the ability of AhR activation to induce highly immunosuppressive cells of the myeloid lineage. Therefore, the ability to control AhR activation is becoming imperative to modulate immunosuppression and inflammation. Recently, nanoparticles (NPs) have been engineered to re-establish tolerance via AhR activation [162]. Together, the success of these studies would provide great promise for AhR as a therapeutic for immunomodulation.
Lipids
Lipid metabolism regulates both differentiation, expansion, and effector function of MDSCs. High-fat diet favours the differentiation of MDSCs from BM-derived precursors and potentiates the suppressive activity of these cells, in mice [163]. In tumour-bearing mice, obesity is associated with an increased accumulation of MDSCs and a reduced CD8+ T cell to MDSC ratio and elevated adiposity is also associated with the accumulation of MDSCs in the spleens and lymph nodes of tumour-free mice. In MDSCs, lipids enter cells by the scavenger receptor CD36, and promote the switch from glycolysis to fatty acid oxidation (FAO), as a primary source of energy [164]. In accordance, the deletion of CD36 or FAO inhibition deprives MDSCs of their immunosuppressive function, delaying tumour growth and enhancing the efficacy of chemotherapy and immunotherapy [165]. Recently, the fatty acid transport protein 2 (FATP2) was identified as a regulator of the suppressive functions of PMN-MDSCs. FATP2 is responsible for arachidonic acid uptake and subsequent PGE2 synthesis. FATP2 inhibition abrogates PMN-MDSC-suppressive functions and enhances cancer immunotherapy efficacy [166].
Glucose
MDSCs rely in glycolysis, the pentose phosphate, and TCA pathways to differentiate and fulfil their functions [15]. Indeed, MDSCs are endowed with a high glucose and glutamine uptake rates, a reduced oxygen consumption rate, and most of their synthesised ATP is obtained through glycolysis-dependent mechanisms [167, 168]. Thus, high glycolytic flux is required for MDSC maturation from bone marrow precursors suggesting an indirect immune suppressive mechanism towards T cells mediated by carbon source consumption. Moreover, the upregulation of glycolytic pathways protects MDSCs from apoptosis and contributes to their survival by preventing ROS-mediated apoptosis via the anti-oxidant activity of the glycolytic intermediate phosphoenolpyruvate. Glycolysis rate is associated with sustained ARG1 activity in MDSCs. Under hypoxic conditions, HIF1α activation triggers the oxidative phosphorylation to glycolysis switch in MDSCs [169]. HIF1α is a critical differentiation and function regulator of MDSCs in the TME [170]. In fact, M-MDSCs show a dormant metabolic state, fail to metabolise glucose, and have a reduced cellular ATP content and low basal mitochondrial respiration [171]. This peculiar metabolic phenotype is regulated by methylglyoxal accumulation in MDSCs, which is then transferred to T lymphocytes. Methylglyoxal suppresses T cell function by the chemical depletion of L-arginine, as well as by rendering L-arginine-containing proteins non-functional through a glycation-dependent mechanism.
Notably, many of these immune suppressive circuits are interconnected and sustained in catalytic loops: for example, metabolic shift from glycolysis to oxidative phosphorylation in myeloid cells increases the production and release of ATP, which in turn acts on CD39, CD73, and adenosine receptors to support an immunosuppressive transcriptional program. According to this, recent clinical trials have been designed in order to evaluate the prominence and safety of specific inhibitors for many enzymes (i.e., IDO, ARG1, NOS2) alone or in combination with target therapy and checkpoint inhibitors (Table 2) [172, 173]. For example, epacadostat, that is a nanomolar inhibitor of IDO1, has been confirmed to be effective and safe when combined with immune checkpoint inhibitors in patients with melanoma (combined with ipilimumab) and those with non-small-cell lung cancer, squamous cell carcinoma of the head and neck, renal cell carcinoma, and urothelial carcinoma (combined with pembrolizumab) in phase I/II. However, a phase III study (NCT02752074) of epacadostat combined with pembrolizumab in patients with unresectable or metastatic melanoma demonstrated that this compound did not enhance anti-PD-1 therapeutic effect [174]. The unexpected results may be the consequence of improper study design, insufficient drug exposure, and/or inappropriate combination strategy. Indeed, enrolled patients were not selected according to the expression of IDO in TME. Guaranteeing sufficient drug exposure, testing new combination protocols, and performing distinctive analysis of primary and secondary endpoints in both IDO positive and negative patients’ subpopulations should be taken into consideration in the future phase III clinical trials. Furthermore, a more precise clarification of the biology of enzyme targets in TME is mandatory to understand where and when these pathways have to be targeted. For instance, IDO1 contains two functional immunoreceptor tyrosine-based inhibitory motifs (ITIMs), which modulate the immune response of IDO1-expressing myeloid cells [175,176,177]. Moreover, IDO1 controls also a multi-pronged anti-ferroptotic death pathway, which plays a pivotal role in tumour suppression in TME [178]. Indeed, IDO1+ cells export kynurenines, which are imported by non-IDO1-expressing cells via solute carrier transporters, and these tryptophan catabolites are converted in metabolites with anti-ferroptotic activity [179], supporting local immunosuppression and cancer cell proliferation. To overcome these substantial limitations linked to the pleiotropic effects of the activity of these enzymes [58], a strategy based of active vaccination can be exploited. This active immunotherapeutic strategy has been also validated in a clinical trial, in which patients with metastatic melanoma were treated with a combinatorial IDO/PD-L1-targeting approach based on IDO1-peptide vaccine combined with nivolumab [180].
Targeting cell-to-cell interaction
Physical interactions influence a bidirectional crosstalk between myeloid cells and different TME cell components, including tumour cells, stromal cells, and immune cells. Amongst cell-to-cell interactions leading to switch off T cell-mediated anti-tumour response, immune checkpoints are crucial inhibitory pathways responsible for cancer immune evasion. MDSCs express both PD-L1, interacting with PD-1 expressed on T cells, and CTLA-4. PD-1 prevents T-cell immune-reactivity via engaging with PD-L1 expressed on tumour/myeloid cells in the “canonical” PD-1/PD-L1 axis. Whilst the effect of the canonical PD-1/PD-L1 axis and its inhibition have been extensively described [181], several evidences regarding the presence of a myeloid-dependent “non-canonical” PD1/PD-L1 function could represent a clear turning point for a reconsideration of PD-1/PD-L1 axis targeting. Indeed, different studies have confirmed PD-1 expression on monocytes, macrophages, DCs, and MDSCs in tumour models and patients [182,183,184,185,186,187]. PD-L1 could be induced on activated T cells and interact with intra-tumoural PD-1 myeloid cells, resulting in various pro-tumour effects [188]. As an example, PD-1 suppressed STAT1- and NF-κB-mediated M1 polarisation promoting M2 polarisation by increasing STAT6 phosphorylation [189, 190]. Recently, Gordon et al. demonstrate that PD-1 expression on TAMs strongly reduces the phagocytic activity against tumour cells [183]. Thus, considering the role of myeloid-PD-1 during ICT, these findings could have important therapeutic implications. The role of myeloid CTLA-4 remains partially unclear. The cis CTLA-4 blockade on T cell has been reported by different authors, but its function in mediating T cell inhibition by MDSCs is still unclear and under investigation [191]. CTLA-4 ligands, such as B7 molecules, are highly expressed by TAMs and DCs in the tumour microenvironment and their expression directly correlates with the reduction of anti-tumour T cell by inhibiting CD28 in several tumour models [192, 193]. Moreover, myeloid cells express as well inhibitory receptors promoting immune suppressive functions, including scavenger receptors as macrophage receptor with collagenous structure (MARCO) and triggering receptor expressed on myeloid cells 2 (TREM-2), common lymphatic endothelial and vascular endothelial receptor 1 (CLEVER), and Ig-like receptors, such as sialic acid-binding immunoglobulin-like lectins (SIGLEC15) and V-domain Ig suppressor of T cell activation (VISTA). These receptors are associated with anti-inflammatory immune suppressive phenotype of myeloid cells which dampens T cell activation and function [194,195,196,197]. Antibodies blocking those receptors recently entered the clinical evaluation phase alone or in combination with ICT (Table 3) and they could open a new age of cancer immunotherapy in the near future. For instance, TREM-2 is an activating receptor of the Ig superfamily that binds lipids and transduces intra-cellular signals through the adaptor DAP12 [198]. DAP12 recruits the protein tyrosine kinase Syk, which initiates a cascade of tyrosine phosphorylation events which lead to the activation of PLCγ2, PI3K, mTOR, and MAPK, ultimately leading to cell activation, metabolic adaptation, and transcriptional rearrangement [199]. TREM-2 is expressed in TAMs and MDSCs [194, 200, 201]. To support its function, a subset of myeloid cells co-expressing ARG1 and TREM-2 were identified in several preclinical models of cancers and genetic ablation of TREM-2 in mice inhibited accumulation of intra-tumour myeloid cells, leading to a decrease in dysfunctional CD8+ T cells and reduced tumour growth [194]. TREM-1 and TREM-2 are expressed on MDSCs and TAMs and correlate with tumour increased volume in preclinical 4T1-breast cancer model. In accordance, high TREM2 expression on tumour myeloid cells is associated with a poor survival rate in patients with colorectal carcinoma or triple-negative breast cancer [201]. Recent reports highlight a novel role for the apolipoprotein E (APOE)-TREM-2 axis in cancer [194, 202], providing promising novel therapeutic targets. TREM-2-deficiency enhances the efficacy of the anti-PD-1 treatment and antibody-dependent TREM-2 blockade is sufficient to remodel the intra-tumour myeloid compartment and to slow tumour growth. On the other hand, expression of some APOE variants, like APOE4, is associated with an improved responsiveness of melanoma patients to anti-PD1 ICT, suggesting that both APOE and TREM-2 expressions on myeloid cells can be used to stratify patients who might benefit from this therapeutic strategy.
Targeting pro-tumour features by myeloid cell-reprogramming
Myeloid cells play a critical role and promptly respond to infections and danger signals and in supporting activation of adaptive immune response towards foreign antigens and pathological conditions (cancer included). In order to finely tune immune system activation and relief, myeloid cells are endowed with both activation and inhibition receptors. Pattern recognition receptors (PRR), such as toll-like receptors (TLR), nod-Like receptors (NLRs), and cytosolic sensors such as stimulator of interferon genes (STING) involved in the sensing of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), are responsible for tuning both myeloid activation and further polarisation of immune response towards an anti-tumour or pro-tumour phenotype [203]. In accordance, many TLRs agonists (imiquimod for TLR7 and CpG for TLR9) or cGAS-STING triggers were employed to activate anti-viral interferon-mediated immune responses with final aim to polarise myeloid cells towards an anti-tumour state, prime, and support T cell-mediated anti-tumour immunity [204, 205]. For instance, monophosphoryl lipid A-mediated TLR4 triggering synergises with IFN-γ to activate a pro-inflammatory type 1 IFN conversion of macrophages isolated from metastatic pleural effusions of breast cancer patients conferring them direct anti-tumour cytotoxic abilities. Moreover, intra-peritoneal or intra-tumour injection of reprogrammed macrophages controls tumour progression and metastatic spreading of breast and ovarian tumours, in preclinical models [206]. Notably, the adoption of PRR agonists can support the infiltration of T lymphocytes in cold tumours, such as pancreatic cancer [204, 207], and synergise with ICT to revert immunotherapy resistance in different preclinical models of solid cancers [208, 209]. Another, non-redundant (PRR independent) strategy to activate myeloid cells to directly reshape TME contexture and sustain a tumour-specific T cell immune response is mediated by CD40 triggering, which is able to support immune response in tumours resistant to immunotherapy such as PDAC, in both mouse models and human patients [210, 211]. These data pave the way to phase 1 clinical evaluation of CD40 agonist with chemotherapy and ICT combination in pancreatic cancer patients which showed clinical activity and deserve further investigation [212]. Nonetheless, the encouraging results, two aspects must be considered for further development of these combinatorial approaches. In first instance, type I and II interferon signalling activates negative feedbacks restraining T cell functions (such as PD-L1 expression on myeloid and tumour cells, PD-1, and CTLA-4 expression on T cells); moreover, TLRs can be expressed by tumour cells as well and those agonists can support their proliferation potential [213, 214]. According to this, another opportunity to promote anti-tumour polarisation of myeloid cells is the employment of integrin agonists. CD11b (ITGAM), a marker shared within myeloid cells, associates with CD18 (ITGB2) to establish Mac-1 or complement receptor 3 (CR3) involved in myeloid trafficking and phagocytosis of opsonised bacteria. Recent investigations proved that CD11b has a crucial role in myeloid activation towards an inflammatory phenotype promoting tumour control in preclinical models of murine and human cancer [215]. ADH-503, a CD11b agonist, is indeed able to repolarise TAM towards an anti-tumour phenotype and to enhance dendritic cell responses which in turn support T cell-mediated tumour restriction and synergism with ICT in pancreatic cancer, a tumour in which ICT does not show any benefit when employed as single agent [67]. Given the promising preclinical results, ADH-503 recently started clinical phase 1/2 evaluation in patients with advanced solid tumour types expected to be resistant to immunotherapy, including pancreatic, prostate, breast, and MS stable colorectal cancers (clinical trial: NCT04060342). Finally, the p53 activation has been reported to promote MDSC differentiation to cross-presenting DCs. Indeed, the pharmacological activation of p53 drives MDSC differentiation to Ly6C+CD103+ DCs, which are essential to enhance a CD8+ T cell anti-tumour immune response during ICT [216]. Taken together, these recent findings pinpoint a novel therapeutic approach to induce immunosuppressive TIM differentiation to antigen-presenting cells rather than causing their elimination.
Conclusion and future perspective
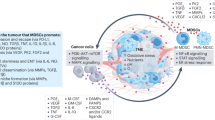
In this review, we have described our current knowledge on targets and strategies set in place to modulate the tumor-promoting function of myeloid cells. However, despite these exciting new opportunities, it is mandatory to keep in mind that all these efforts are meaningful if successfully translated to humans. In this contest, it will be important to include analysis of BM niches in which to explore potential new targets regarding MDSC generation, regulation, and trafficking. We think that targeting the BM niches presents not only an avenue to treat cancer but also inflammatory conditions, since emergency myelopoiesis is a highly regulated process in which HSC niche and external factors tilt the hematopoiesis balance towards an altered myeloid lineage. The pivotal work done by several research groups, on defining and understanding the regulatory elements sustaining HSC output, provides promising molecular targets that could potentially revert a “maladaptive” myelopoiesis into an educated one (Fig. 2). Today, single-cell omic technologies are improving the current understanding of myeloid cell biology and their contribution to tumour progression and tumour restriction. Preclinical and clinical studies highlight the importance of re-educating in spite of depleting specific myeloid cell subsets within TME in order to sustain anti-tumour immunity. From this point of view, many strategies can be enrolled to build up a new concept of cancer immunotherapy.
Cartoon depicting the dynamics of tumor-induced emergency myelopoiesis. Tumor microenvironment (TME)-derived soluble and bioactive factors (cytokines, growth factors, exosomes, nanoparticles, cells) condition the BM to output corrupted altered myeloid cells (emergency myelopoiesis) which promote primary cancer growth and metastatic spread (lung and lymph nodes). Hematopoietic stem cells (HSCs), in the so-called BM niche, interact with mesenchymal (MSCs) and endothelial cells that regulate HSC dormancy and differentiation into altered progenitors through cytokines and cell contact-dependent signals. Several mechanisms (depicted by dashed lines) will be the focus of future investigations. From these studies, new targets will be identified and exploited for alternative, more effective, and personalised therapeutic approaches for cancer disease
Recently, myeloid cell engineering by gene-editing approaches has been proposed as well to sustain antigen presentation and tumour cytotoxic activities. The manipulation of myeloid immunity has some advantages compared to T cell engineering. In first instance, myeloid cells can infiltrate TME more efficiently than lymphocytes. Secondly, engineered myeloid cells can shape TME towards a tumour restricting milieu, support priming of tumour-specific immune response, homing, and function of T lymphocytes in tumour core. For example, monocytes engineered to express a pp65 (CMV) protein fused to lysosomal-associated membrane protein (LAMP) were adoptively transferred in glioblastoma patients in order to efficiently prime a tumour-specific T cell-based immune response (clinical trial: NCT04741984). IL-12 and type I and II IFNs promote myeloid cell skewing towards an inflammatory phenotype: genetically engineered myeloid cells expressing IL-12 reverted the immune suppressive program in the premetastatic niche supporting tumour antigen priming and resulting in reduced metastatic and primary tumour burden in tumour-bearing mice [217]. Similar results were achieved in preclinical breast cancer setting employing IFN-γ-loaded macrophages [218]. Finally, macrophages engineered with a epidermal growth factor receptor 2 (Her2)-specific chimeric-antigen receptor showed deepen abilities to phagocyte cancer cells and secrete pro-inflammatory cytokines supporting the M1-like polarisation of bystander myeloid cells, restricting tumour burden [219].
The specific and efficient delivery of modulators to tumour-reprogrammed myeloid cells can improve the efficacy of cancer therapy. Nanoparticles (NPs) are thus excellent candidates to modulate TME-infiltrating myeloid cells [220]. NPs are carriers of any shape which size ranges between 1 and 100 nm with distinctive features for immune cell targeting such as the ability to overcome biological barriers and to be engulfed by immune cells [221]. Multiple factors impact the effectiveness of the NP-based therapy, such as (i) route of administration, (ii) particle surface charge, and (iii) drug formulation. For instance, MDSC/TAM-targeted NPs are normally infused intravenously and can accumulate passively or as consequence of myeloid cell uptake in tumours. However, systemic administration provides liver, kidney, and spleen accumulation that compromise a preferential uptake by tumour-infiltrated MDSCs/TAMs. To avoid this important limitation, several studies have developed NP-based targeting systems employing surface markers to specifically target defined immune cell subsets [222]. For instance, polyethylene glycol (PEG)-sheddable, mannose-modified NPs were developed to target M2-like TAMs via mannose-CD206 binding after pH-sensitive PEG dissociation in the acidic TME [223]. Poly-beta-amino-ester (PBAE) NPs as cargo of synthetic mRNA encoding interferon regulatory factor 5 (IRF5) was able to affect M2-like TAMs and increase the percentage of M1-like TAMs [224]. A similar TAM reprogramming is promoted also by IL-12-loaded poly-β-amino ester NPs [225]. Other NPs can be loaded with silencing molecules (i.e. shRNA, siRNA) to target crucial transcriptional factors in myeloid, reprogrammed cells such as STAT3. Indeed, the STAT3-silencing inhibited MDSC-dependent immunosuppression at the tumour site in tumour-bearing mice [226] as well as normalised the immune response by repressing plasma concentration of several pro-inflammatory cytokines in mice undergoing CRS [59]. Therefore, this approach may be tested in combination with ICT in tumour setting.
In conclusion, although we still do not completely understand the mechanisms driving innate myeloid cell polarisation towards an anti-tumour phenotype, deciphering the myeloid cell functional stages associated with worse clinical outcome and bad response to therapy will support clinicians to select the patients requiring TME reprogramming, increasing thus the therapeutic effectiveness of ICT. Strategies harnessing T cell functions and myeloid cell reprogramming will synergise in supporting immune system ability to restrict tumour progression in the next future.
References
Bagchi S, Yuan R, Engleman EG (2021) Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol 16:223–249
Morad G, Helmink BA, Sharma P, Wargo JA (2021) Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184:5309–5337
Sharma P, Allison JP (2020) Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol 20:75–76
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A (2017) Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168:707–723
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8:1069–1086
Schumacher TN, Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science 348:69–74
Snahnicanova Z, Kasubova I, Kalman M, Grendar M, Mikolajcik P, Gabonova E, Laca L, Caprnda M, Rodrigo L, Ciccocioppo R, Kruzliak P, Plank L, Lasabova Z (2020) Genetic and epigenetic analysis of the beta-2-microglobulin gene in microsatellite instable colorectal cancer. Clin Exp Med 20:87–95
Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P (2016) Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167:397-404 e9
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571
Pitt JM, Vetizou M, Daillere R, Roberti MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M, Kroemer G, Zitvogel L (2016) Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44:1255–1269
Kumar V, Patel S, Tcyganov E, Gabrilovich DI (2016) The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 37:208–220
De Sanctis F, Bronte V, Ugel S (2016) Tumor-induced myeloid-derived suppressor cells. Microbiol Spectr 4(3). https://doi.org/10.1128/microbiolspec.MCHD-0016-2015
Trovato R, Cane S, Petrova V, Sartoris S, Ugel S, De Sanctis F (2020) The engagement between MDSCs and metastases: partners in crime. Front Oncol 10:165
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V (2012) Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12:253–268
Veglia F, Perego M, Gabrilovich D (2018) Myeloid-derived suppressor cells coming of age. Nat Immunol 19:108–119
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355
Hanahan D (2022) Hallmarks of cancer: new dimensions. Cancer Discov 12:31–46
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24:541–550
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661
Sica A, Guarneri V, Gennari A (2019) Myelopoiesis, metabolism and therapy: a crucial crossroads in cancer progression. Cell Stress 3:284–294
Ugel S, Cane S, De Sanctis F, Bronte V (2021) Monocytes in the tumor microenvironment. Annu Rev Pathol 16:93–122
Long H, Jia Q, Wang L, Fang W, Wang Z, Jiang T, Zhou F, Jin Z, Huang J, Zhou L, Hu C, Wang X, Zhang J, Ba Y, Gong Y, Zeng X, Zeng D, Su X, Alexander PB, Wang L, Wang L, Wan YY, Wang XF, Zhang L, Li QJ, Zhu B (2022) Tumor-induced erythroid precursor-differentiated myeloid cells mediate immunosuppression and curtail anti-PD-1/PD-L1 treatment efficacy. Cancer Cell 40:674–93 e7
Ugel S, De Sanctis F, Mandruzzato S, Bronte V (2015) Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest 125:3365–3376
Hegde S, Leader AM, Merad M (2021) MDSC: Markers, development, states, and unaddressed complexity. Immunity 54:875–884
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D (2020) Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20:7–24
Engblom C, Pfirschke C, Pittet MJ (2016) The role of myeloid cells in cancer therapies. Nat Rev Cancer 16:447–462
Coffelt SB, Wellenstein MD, de Visser KE (2016) Neutrophils in cancer: neutral no more. Nat Rev Cancer 16:431–446
Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P (2019) Neutrophil diversity in health and disease. Trends Immunol 40:565–583
Mantovani A, Marchesi F, Jaillon S, Garlanda C, Allavena P (2021) Tumor-associated myeloid cells: diversity and therapeutic targeting. Cell Mol Immunol 18:566–578
Mantovani A, Sica A (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 22:231–237
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41:14–20
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Di Caro G, Cortese N, Castino GF, Grizzi F, Gavazzi F, Ridolfi C, Capretti G, Mineri R, Todoric J, Zerbi A, Allavena P, Mantovani A, Marchesi F (2016) Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut 65:1710–1720
Marigo I, Trovato R, Hofer F, Ingangi V, Desantis G, Leone K, De Sanctis F, Ugel S, Cane S, Simonelli A, Lamolinara A, Iezzi M, Fassan M, Rugge M, Boschi F, Borile G, Eisenhaure T, Sarkizova S, Lieb D, Hacohen N, Azzolin L, Piccolo S, Lawlor R, Scarpa A, Carbognin L, Bria E, Bicciato S, Murray PJ, Bronte V (2020) Disabled homolog 2 controls prometastatic activity of tumor-associated macrophages. Cancer Discov 10:1758–1773
DeNardo DG, Ruffell B (2019) Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol 19:369–382
Cassetta L, Pollard JW (2018) Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 17:887–904
Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G (2017) The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14:717–734
Ruffell B, Coussens LM (2015) Macrophages and therapeutic resistance in cancer. Cancer Cell 27:462–472
Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guerin M, Biton J, Ouakrim H, Regnier F, Lupo A, Alifano M, Damotte D, Donnadieu E (2018) Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci U S A 115:E4041–E4050
Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, Pittet MJ (2017) In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med 9(389):eaal3604. https://doi.org/10.1126/scitranslmed.aal3604
Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, Partlova S, Garfall A, Vogl DT, Xu X, Knight SC, Malietzis G, Lee GH, Eruslanov E, Albelda SM, Wang X, Mehta JL, Bewtra M, Rustgi A, Hockstein N, Witt R, Masters G, Nam B, Smirnov D, Sepulveda MA, Gabrilovich DI (2016) Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol 1(2):aaf8943. https://doi.org/10.1126/sciimmunol.aaf8943
Veglia F, Sanseviero E, Gabrilovich DI (2021) Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol 21:485–498
Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, Nee K, Hernandez G, Evans K, Torosian L, Silva A, Walsh C, Kessenbrock K (2020) Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci Immunol 5(44):eaay6017. https://doi.org/10.1126/sciimmunol.aay6017
Moses K, Brandau S (2016) Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol 28:187–196
Darcy CJ, Minigo G, Piera KA, Davis JS, McNeil YR, Chen Y, Volkheimer AD, Weinberg JB, Anstey NM, Woodberry T (2014) Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care 18:R163
Bost P, De Sanctis F, Cane S, Ugel S, Donadello K, Castellucci M, Eyal D, Fiore A, Anselmi C, Barouni RM, Trovato R, Caligola S, Lamolinara A, Iezzi M, Facciotti F, Mazzariol A, Gibellini D, De Nardo P, Tacconelli E, Gottin L, Polati E, Schwikowski B, Amit I, Bronte V (2021) Deciphering the state of immune silence in fatal COVID-19 patients. Nat Commun 12:1428
Schulte-Schrepping J, Reusch N, Paclik D, Bassler K, Schlickeiser S, Zhang B, Kramer B, Krammer T, Brumhard S, Bonaguro L, De Domenico E, Wendisch D, Grasshoff M, Kapellos TS, Beckstette M, Pecht T, Saglam A, Dietrich O, Mei HE, Schulz AR, Conrad C, Kunkel D, Vafadarnejad E, Xu CJ, Horne A, Herbert M, Drews A, Thibeault C, Pfeiffer M, Hippenstiel S, Hocke A, Muller-Redetzky H, Heim KM, Machleidt F, Uhrig A, Bosquillon de Jarcy L, Jurgens L, Stegemann M, Glosenkamp CR, Volk HD, Goffinet C, Landthaler M, Wyler E, Georg P, Schneider M, Dang-Heine C, Neuwinger N, Kappert K, Tauber R, Corman V, Raabe J, Kaiser KM, Vinh MT, Rieke G, Meisel C, Ulas T, Becker M, Geffers R, Witzenrath M, Drosten C, Suttorp N, von Kalle C, Kurth F, Handler K, Schultze JL, Aschenbrenner AC, Li Y, Nattermann J, Sawitzki B, Saliba AE, Sander LE, Deutsche C-OI (2020) Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 182:1419–40 e23
Kostlin N, Vogelmann M, Spring B, Schwarz J, Feucht J, Hartel C, Orlikowsky TW, Poets CF, Gille C (2017) Granulocytic myeloid-derived suppressor cells from human cord blood modulate T-helper cell response towards an anti-inflammatory phenotype. Immunology 152:89–101
Marini O, Costa S, Bevilacqua D, Calzetti F, Tamassia N, Spina C, De Sabata D, Tinazzi E, Lunardi C, Scupoli MT, Cavallini C, Zoratti E, Tinazzi I, Marchetta A, Vassanelli A, Cantini M, Gandini G, Ruzzenente A, Guglielmi A, Missale F, Vermi W, Tecchio C, Cassatella MA, Scapini P (2017) Mature CD10(+) and immature CD10(−) neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood 129:1343–1356
Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V (2010) Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 32:790–802
Kwak T, Wang F, Deng H, Condamine T, Kumar V, Perego M, Kossenkov A, Montaner LJ, Xu X, Xu W, Zheng C, Schuchter LM, Amaravadi RK, Mitchell TC, Karakousis GC, Mulligan C, Nam B, Masters G, Hockstein N, Bennett J, Nefedova Y, Gabrilovich DI (2020) Distinct populations of immune-suppressive macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell Rep 33:108571
Halaby MJ, Hezaveh K, Lamorte S, Ciudad MT, Kloetgen A, MacLeod BL, Guo M, Chakravarthy A, Medina TDS, Ugel S, Tsirigos A, Bronte V, Munn DH, Pugh TJ, De Carvalho DD, Butler MO, Ohashi PS, Brooks DG, McGaha TL (2019) GCN2 drives macrophage and MDSC function and immunosuppression in the tumor microenvironment. Sci Immunol 4(42):eaax8189. https://doi.org/10.1126/sciimmunol.aax8189
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150
Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H, Pardoll D, Kim Y (2013) STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest 123:1580–1589
Trovato R, Fiore A, Sartori S, Cane S, Giugno R, Cascione L, Paiella S, Salvia R, De Sanctis F, Poffe O, Anselmi C, Hofer F, Sartoris S, Piro G, Carbone C, Corbo V, Lawlor R, Solito S, Pinton L, Mandruzzato S, Bassi C, Scarpa A, Bronte V, Ugel S (2019) Immunosuppression by monocytic myeloid-derived suppressor cells in patients with pancreatic ductal carcinoma is orchestrated by STAT3. J Immunother Cancer 7:255
Sangaletti S, Talarico G, Chiodoni C, Cappetti B, Botti L, Portararo P, Gulino A, Consonni FM, Sica A, Randon G, Di Nicola M, Tripodo C, Colombo MP (2019) SPARC is a new myeloid-derived suppressor cell marker licensing suppressive activities. Front Immunol 10:1369
Fiore A, Ugel S, De Sanctis F, Sandri S, Fracasso G, Trovato R, Sartoris S, Solito S, Mandruzzato S, Vascotto F, Hippen KL, Mondanelli G, Grohmann U, Piro G, Carbone C, Melisi D, Lawlor RT, Scarpa A, Lamolinara A, Iezzi M, Fassan M, Bicciato S, Blazar BR, Sahin U, Murray PJ, Bronte V (2018) Induction of immunosuppressive functions and NF-kappaB by FLIP in monocytes. Nat Commun 9:5193
Adamo A, Frusteri C, Pallotta MT, Pirali T, Sartoris S, Ugel S (2020) Moonlighting proteins are important players in cancer immunology. Front Immunol 11:613069
Musiu C, Caligola S, Fiore A, Lamolinara A, Frusteri C, Del Pizzo FD, De Sanctis F, Cane S, Adamo A, Hofer F, Barouni RM, Grilli A, Zilio S, Serafini P, Tacconelli E, Donadello K, Gottin L, Polati E, Girelli D, Polidoro I, Iezzi PA, Angelucci D, Capece A, Chen Y, Shi ZL, Murray PJ, Chilosi M, Amit I, Bicciato S, Iezzi M, Bronte V, Ugel S (2022) Fatal cytokine release syndrome by an aberrant FLIP/STAT3 axis. Cell Death Differ 29:420–438
Haverkamp JM, Smith AM, Weinlich R, Dillon CP, Qualls JE, Neale G, Koss B, Kim Y, Bronte V, Herold MJ, Green DR, Opferman JT, Murray PJ (2014) Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways. Immunity 41:947–959
Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, Choi K, Fromme RM, Dao P, McKenney PT, Wasti RC, Kadaveru K, Mazutis L, Rudensky AY, Pe’er D (2018) Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174:1293–308 e36
Caligola S, De Sanctis F, Cane S, Ugel S (2022) Breaking the immune complexity of the tumor microenvironment using single-cell technologies. Front Genet 13:867880
Sanin DE, Ge Y, Marinkovic E, Kabat AM, Castoldi A, Caputa G, Grzes KM, Curtis JD, Thompson EA, Willenborg S, Dichtl S, Reinhardt S, Dahl A, Pearce EL, Eming SA, Gerbaulet A, Roers A, Murray PJ, Pearce EJ (2022) A common framework of monocyte-derived macrophage activation. Sci Immunol 7:eabl7482
Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, Qin S, Zhang L, Ouyang H, Du P, Jiang L, Zhang B, Yang Y, Wang X, Ren X, Bei JX, Hu X, Bu Z, Ji J, Zhang Z (2021) A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184:792-809 e23
Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z, Ding X, Bao R, Hong L, Jia W, Fang F, Liu H, Chen L, Zhong J, Zou D, Liu L, Han L, Ginhoux F, Liu Y, Ye Y, Su B (2022) Single-cell and spatial analysis reveal interaction of FAP(+) fibroblasts and SPP1(+) macrophages in colorectal cancer. Nat Commun 13:1742
Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O’Brien SA, He Y, Wang L, Zhang Q, Kim A, Gao R, Orf J, Wang T, Sawant D, Kang J, Bhatt D, Lu D, Li CM, Rapaport AS, Perez K, Ye Y, Wang S, Hu X, Ren X, Ouyang W, Shen Z, Egen JG, Zhang Z, Yu X (2020) Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181:442–59 e29
Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg GD, Knolhoff BL, Breden MA, Li X, Krisnawan VE, Khan SQ, Schwarz JK, Rogers BE, Fields RC, Hawkins WG, Gupta V, DeNardo DG (2019) Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci Transl Med 11(499):eaau9240. https://doi.org/10.1126/scitranslmed.aau9240
Zhang H, Nguyen-Jackson H, Panopoulos AD, Li HS, Murray PJ, Watowich SS (2010) STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood 116:2462–2471
Li T, Li X, Zamani A, Wang W, Lee CN, Li M, Luo G, Eiler E, Sun H, Ghosh S, Jin J, Murali R, Ruan Q, Shi W, Chen YH (2020) c-Rel is a myeloid checkpoint for cancer immunotherapy. Nat Cancer 1:507–517
Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, Schmid MC, Pink M, Winkler DG, Rausch M, Palombella VJ, Kutok J, McGovern K, Frazer KA, Wu X, Karin M, Sasik R, Cohen EE, Varner JA (2016) PI3Kgamma is a molecular switch that controls immune suppression. Nature 539:437–442
Abad C, Nobuta H, Li J, Kasai A, Yong WH, Waschek JA (2014) Targeted STAT3 disruption in myeloid cells alters immunosuppressor cell abundance in a murine model of spontaneous medulloblastoma. J Leukoc Biol 95:357–367
Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, Schmid MC, Sun P, Mose E, Bouvet M, Lowy AM, Valasek MA, Sasik R, Novelli F, Hirsch E, Varner JA (2016) Macrophage PI3Kgamma drives pancreatic ductal adenocarcinoma progression. Cancer Discov 6:870–885
Johnson DE, O’Keefe RA, Grandis JR (2018) Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 15:234–248
Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, Ochoa AC, Cui Y, Del Valle L, Rodriguez PC (2014) The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity 41:389–401
Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205:2235–2249
Netherby CS, Messmer MN, Burkard-Mandel L, Colligan S, Miller A, Cortes Gomez E, Wang J, Nemeth MJ, Abrams SI (2017) The granulocyte progenitor stage is a key target of IRF8-mediated regulation of myeloid-derived suppressor cell production. J Immunol 198:4129–4139
Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, Abrams SI (2013) Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest 123:4464–4478
Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, Hockstein N, Guarino M, Masters G, Penman E, Denstman F, Xu X, Altieri DC, Du H, Yan C, Gabrilovich DI (2016) CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity 44:303–315
Law AMK, Valdes-Mora F, Gallego-Ortega D (2020) Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells 9(3):561. https://doi.org/10.3390/cells9030561
Huang S, Wang Z, Zhou J, Huang J, Zhou L, Luo J, Wan YY, Long H, Zhu B (2019) EZH2 inhibitor GSK126 suppresses antitumor immunity by driving production of myeloid-derived suppressor cells. Cancer Res 79:2009–2020
Wang HF, Ning F, Liu ZC, Wu L, Li ZQ, Qi YF, Zhang G, Wang HS, Cai SH, Du J (2017) Histone deacetylase inhibitors deplete myeloid-derived suppressor cells induced by 4T1 mammary tumors in vivo and in vitro. Cancer Immunol Immunother 66:355–366
Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, Sarnaik A, Horna P, Sotomayor E, Gabrilovich DI (2013) Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol 14:211–220
Sahakian E, Powers JJ, Chen J, Deng SL, Cheng F, Distler A, Woods DM, Rock-Klotz J, Sodre AL, Youn JI, Woan KV, Villagra A, Gabrilovich D, Sotomayor EM, Pinilla-Ibarz J (2015) Histone deacetylase 11: a novel epigenetic regulator of myeloid derived suppressor cell expansion and function. Mol Immunol 63:579–585
de Almeida Nagata DE, Chiang EY, Jhunjhunwala S, Caplazi P, Arumugam V, Modrusan Z, Chan E, Merchant M, Jin L, Arnott D, Romero FA, Magnuson S, Gascoigne KE, Grogan JL (2019) Regulation of tumor-associated myeloid cell activity by CBP/EP300 bromodomain modulation of H3K27 acetylation. Cell Rep 27:269–81 e4
Xin J, Zhang Z, Su X, Wang L, Zhang Y, Yang R (2017) Epigenetic component p66a modulates myeloid-derived suppressor cells by modifying STAT3. J Immunol 198:2712–2720
Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, Chintala S, Ordentlich P, Kao C, Elzey B, Gabrilovich D, Pili R (2017) Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res 23:5187–5201
Hashimoto A, Fukumoto T, Zhang R, Gabrilovich D (2020) Selective targeting of different populations of myeloid-derived suppressor cells by histone deacetylase inhibitors. Cancer Immunol Immunother 69:1929–1936
Daurkin I, Eruslanov E, Vieweg J, Kusmartsev S (2010) Generation of antigen-presenting cells from tumor-infiltrated CD11b myeloid cells with DNA demethylating agent 5-aza-2′-deoxycytidine. Cancer Immunol Immunother 59:697–706
Rodriguez-Ubreva J, Catala-Moll F, Obermajer N, Alvarez-Errico D, Ramirez RN, Company C, Vento-Tormo R, Moreno-Bueno G, Edwards RP, Mortazavi A, Kalinski P, Ballestar E (2017) Prostaglandin E2 leads to the acquisition of DNMT3A-dependent tolerogenic functions in human myeloid-derived suppressor cells. Cell Rep 21:154–167
Zhou J, Shen Q, Lin H, Hu L, Li G, Zhang X (2019) Decitabine shows potent anti-myeloma activity by depleting monocytic myeloid-derived suppressor cells in the myeloma microenvironment. J Cancer Res Clin Oncol 145:329–336
Zhou J, Yao Y, Shen Q, Li G, Hu L, Zhang X (2017) Demethylating agent decitabine disrupts tumor-induced immune tolerance by depleting myeloid-derived suppressor cells. J Cancer Res Clin Oncol 143:1371–1380
Lu Z, Zou J, Li S, Topper MJ, Tao Y, Zhang H, Jiao X, Xie W, Kong X, Vaz M, Li H, Cai Y, Xia L, Huang P, Rodgers K, Lee B, Riemer JB, Day CP, Yen RC, Cui Y, Wang Y, Wang Y, Zhang W, Easwaran H, Hulbert A, Kim K, Juergens RA, Yang SC, Battafarano RJ, Bush EL, Broderick SR, Cattaneo SM, Brahmer JR, Rudin CM, Wrangle J, Mei Y, Kim YJ, Zhang B, Wang KK, Forde PM, Margolick JB, Nelkin BD, Zahnow CA, Pardoll DM, Housseau F, Baylin SB, Shen L, Brock MV (2020) Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature 579:284–290
Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V (2012) Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2:628–639
Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW (2015) CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med 212:1043–1059
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475:222–225
Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H (2017) Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66:157–167
Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y, Wang F, Duan X, Li J, Zhang W, Wang H (2017) A natural CCR2 antagonist relieves tumor-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer. EBioMedicine 22:58–67
Fei L, Ren X, Yu H, Zhan Y (2021) Targeting the CCL2/CCR2 axis in cancer immunotherapy: one stone, three birds? Front Immunol 12:771210
Teng KY, Han J, Zhang X, Hsu SH, He S, Wani NA, Barajas JM, Snyder LA, Frankel WL, Caligiuri MA, Jacob ST, Yu J, Ghoshal K (2017) Blocking the CCL2-CCR2 axis using CCL2-neutralizing antibody is an effective therapy for hepatocellular cancer in a mouse model. Mol Cancer Ther 16:312–322
Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M (2014) Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 515:130–133
Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, Li S, Seetharam S, Puchalski TA, Takimoto C, Elsayed Y, Dawkins F, de Bono JS (2013) Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs 31:760–768
Walens A, DiMarco AV, Lupo R, Kroger BR, Damrauer JS, Alvarez JV (2019) CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. Elife 8:e43653. https://doi.org/10.7554/eLife.43653
Delprat V, Michiels C (2021) A bi-directional dialog between vascular cells and monocytes/macrophages regulates tumor progression. Cancer Metastasis Rev 40:477–500
Reichel CA, Puhr-Westerheide D, Zuchtriegel G, Uhl B, Berberich N, Zahler S, Wymann MP, Luckow B, Krombach F (2012) C-C motif chemokine CCL3 and canonical neutrophil attractants promote neutrophil extravasation through common and distinct mechanisms. Blood 120:880–890
Zilio S, Bicciato S, Weed D, Serafini P (2022) CCR1 and CCR5 mediate cancer-induced myelopoiesis and differentiation of myeloid cells in the tumor. J Immunother Cancer 10(1):e003131. https://doi.org/10.1136/jitc-2021-003131
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJ, Kaminker JS, Modrusan Z, van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A 107:21248–21255
Belkin DA, Mitsui H, Wang CQ, Gonzalez J, Zhang S, Shah KR, Coats I, Suarez-Farinas M, Krueger JG, Felsen D, Carucci JA (2013) CD200 upregulation in vascular endothelium surrounding cutaneous squamous cell carcinoma. JAMA Dermatol 149:178–186
Choueiry F, Torok M, Shakya R, Agrawal K, Deems A, Benner B, Hinton A, Shaffer J, Blaser BW, Noonan AM, Williams TM, Dillhoff M, Conwell DL, Hart PA, Cruz-Monserrate Z, Bai XF, Carson WE 3rd, Mace TA (2020) CD200 promotes immunosuppression in the pancreatic tumor microenvironment. J Immunother Cancer 8(1):e000189. https://doi.org/10.1136/jitc-2019-000189
Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL, Li CW, Lim SO, Sheng YY, Zhang Y, Li JH, Luo Q, Zheng Y, Zhao Y, Lu L, Jia HL, Hung MC, Dong QZ, Qin LX (2019) Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 68:1653–1666
Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S (2009) Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol 183:937–944
Ku AW, Muhitch JB, Powers CA, Diehl M, Kim M, Fisher DT, Sharda AP, Clements VK, O’Loughlin K, Minderman H, Messmer MN, Ma J, Skitzki JJ, Steeber DA, Walcheck B, Ostrand-Rosenberg S, Abrams SI, Evans SS (2016) Tumor-induced MDSC act via remote control to inhibit L-selectin-dependent adaptive immunity in lymph nodes. Elife 5:e17375. https://doi.org/10.7554/eLife.17375
Mehta HM, Malandra M, Corey SJ (2015) G-CSF and GM-CSF in neutropenia. J Immunol 195:1341–1349
Takeuchi S, Baghdadi M, Tsuchikawa T, Wada H, Nakamura T, Abe H, Nakanishi S, Usui Y, Higuchi K, Takahashi M, Inoko K, Sato S, Takano H, Shichinohe T, Seino K, Hirano S (2015) Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res 75:2629–2640
Kast RE, Hill QA, Wion D, Mellstedt H, Focosi D, Karpel-Massler G, Heiland T, Halatsch ME (2017) Glioblastoma-synthesized G-CSF and GM-CSF contribute to growth and immunosuppression: potential therapeutic benefit from dapsone, fenofibrate, and ribavirin. Tumour Biol 39:1010428317699797
Weber R, Groth C, Lasser S, Arkhypov I, Petrova V, Altevogt P, Utikal J, Umansky V (2021) IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol 359:104254
Chen CC, Chen WC, Lu CH, Wang WH, Lin PY, Lee KD, Chen MF (2010) Significance of interleukin-6 signaling in the resistance of pharyngeal cancer to irradiation and the epidermal growth factor receptor inhibitor. Int J Radiat Oncol Biol Phys 76:1214–1224
Tannenbaum CS, Rayman PA, Pavicic PG, Kim JS, Wei W, Polefko A, Wallace W, Rini BI, Morris-Stiff G, Allende DS, Hamilton T, Finke JH, Diaz-Montero CM (2019) Mediators of inflammation-driven expansion, trafficking, and function of tumor-infiltrating MDSCs. Cancer Immunol Res 7:1687–1699
Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V (2015) Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer 136:2352–2360
Shi H, Zhang J, Han X, Li H, Xie M, Sun Y, Liu W, Ba X, Zeng X (2017) Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1beta-mediated increase in E-selectin expression. Int J Cancer 140:1370–1383
Guo B, Fu S, Zhang J, Liu B, Li Z (2016) Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci Rep 6:36107
Sota J, Vitale A, Insalaco A, Sfriso P, Lopalco G, Emmi G, Cattalini M, Manna R, Cimaz R, Priori R, Talarico R, de Marchi G, Frassi M, Gallizzi R, Soriano A, Alessio M, Cammelli D, Maggio MC, Gentileschi S, Marcolongo R, La Torre F, Fabiani C, Colafrancesco S, Ricci F, Galozzi P, Viapiana O, Verrecchia E, Pardeo M, Cerrito L, Cavallaro E, Olivieri AN, Paolazzi G, Vitiello G, Maier A, Silvestri E, Stagnaro C, Valesini G, Mosca M, de Vita S, Tincani A, Lapadula G, Frediani B, De Benedetti F, Iannone F, Punzi L, Salvarani C, Galeazzi M, Angotti R, Messina M, Tosi GM, Rigante D, Cantarini L, Working Group of Systemic Autoinflammatory Diseases of SIR (2018) Safety profile of the interleukin-1 inhibitors anakinra and canakinumab in real-life clinical practice: a nationwide multicenter retrospective observational study. Clin Rheumatol 37:2233–2240
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E (2018) Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17:588–606
Gehad AE, Lichtman MK, Schmults CD, Teague JE, Calarese AW, Jiang Y, Watanabe R, Clark RA (2012) Nitric oxide-producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas. J Invest Dermatol 132:2642–2651
Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA (2003) Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med 198:1741–1752
Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP (2011) Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 9:e1001162
Younis RH, Han KL, Webb TJ (2016) Human head and neck squamous cell carcinoma-associated semaphorin 4D induces expansion of myeloid-derived suppressor cells. J Immunol 196:1419–1429
Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F (2008) A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135:234–243
Song C, Yuan Y, Wang XM, Li D, Zhang GM, Huang B, Feng ZH (2014) Passive transfer of tumour-derived MDSCs inhibits asthma-related airway inflammation. Scand J Immunol 79:98–104
Ferrara N, Adamis AP (2016) Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov 15:385–403
Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, Ng IO, Wong CC (2017) Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun 8:517
Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M (2013) Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 24:695–709
Henze AT, Mazzone M (2016) The impact of hypoxia on tumor-associated macrophages. J Clin Invest 126:3672–3679
Hofer F, Di Sario G, Musiu C, Sartoris S, De Sanctis F, Ugel S (2021) A complex metabolic network confers immunosuppressive functions to myeloid-derived suppressor cells (MDSCs) within the tumour microenvironment. Cells 10(10):2700. https://doi.org/10.3390/cells10102700
Geeraerts X, Bolli E, Fendt SM, Van Ginderachter JA (2017) Macrophage metabolism as therapeutic target for cancer, atherosclerosis, and obesity. Front Immunol 8:289
Grohmann U, Mondanelli G, Belladonna ML, Orabona C, Pallotta MT, Iacono A, Puccetti P, Volpi C (2017) Amino-acid sensing and degrading pathways in immune regulation. Cytokine Growth Factor Rev 35:37–45
Munn DH, Bronte V (2016) Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol 39:1–6
Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC (2004) Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 64:5839–5849
Van de Velde LA, Guo XJ, Barbaric L, Smith AM, Oguin TH 3rd, Thomas PG, Murray PJ (2016) Stress kinase GCN2 controls the proliferative fitness and trafficking of cytotoxic T cells independent of environmental amino acid sensing. Cell Rep 17:2247–2258
Rodriguez PC, Quiceno DG, Ochoa AC (2007) L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109:1568–1573
Miska J, Rashidi A, Lee-Chang C, Gao P, Lopez-Rosas A, Zhang P, Burga R, Castro B, Xiao T, Han Y, Hou D, Sampat S, Cordero A, Stoolman JS, Horbinski CM, Burns M, Reshetnyak YK, Chandel NS, Lesniak MS (2021) Polyamines drive myeloid cell survival by buffering intracellular pH to promote immunosuppression in glioblastoma. Sci Adv 7(8):eabc8929. https://doi.org/10.1126/sciadv.abc8929
Puleston DJ, Buck MD, Klein Geltink RI, Kyle RL, Caputa G, O’Sullivan D, Cameron AM, Castoldi A, Musa Y, Kabat AM, Zhang Y, Flachsmann LJ, Field CS, Patterson AE, Scherer S, Alfei F, Baixauli F, Austin SK, Kelly B, Matsushita M, Curtis JD, Grzes KM, Villa M, Corrado M, Sanin DE, Qiu J, Pallman N, Paz K, Maccari ME, Blazar BR, Mittler G, Buescher JM, Zehn D, Rospert S, Pearce EJ, Balabanov S, Pearce EL (2019) Polyamines and eIF5A hypusination modulate mitochondrial respiration and macrophage activation. Cell Metab 30:352–63 e8
Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P (2003) L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol 24:302–306
Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM (2002) Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol 168:689–695
Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, Ochoa AC, Fletcher M, Velasco C, Wilk A, Reiss K, Rodriguez PC (2014) Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer 134:2853–2864
De Sanctis F, Lamolinara A, Boschi F, Musiu C, Caligola S, Trovato R, Fiore A, Frusteri C, Anselmi C, Poffe O, Cestari T, Cane S, Sartoris S, Giugno R, Del Rosario G, Zappacosta B, Del Pizzo F, Fassan M, Dugnani E, Piemonti L, Bottani E, Decimo I, Paiella S, Salvia R, Lawlor RT, Corbo V, Park Y, Tuveson DA, Bassi C, Scarpa A, Iezzi M, Ugel S, Bronte V (2022) Interrupting the nitrosative stress fuels tumor-specific cytotoxic T lymphocytes in pancreatic cancer. J Immunother Cancer 10(1):e003549. https://doi.org/10.1136/jitc-2021-003549
Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, Savino B, Colombo P, Jonjic N, Pecanic S, Lazzarato L, Fruttero R, Gasco A, Bronte V, Viola A (2011) Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med 208:1949–1962
Cali B, Agnellini AHR, Cioccarelli C, Sanchez-Rodriguez R, Predonzani A, Toffolo GI, Viola A, Bronte V, Arrigoni G, Zonta F, Albertoni L, Mescoli C, Marigo I, Molon B (2021) GM-CSF nitration is a new driver of myeloid suppressor cell activity in tumors. Front Immunol 12:718098
De Sanctis F, Sandri S, Ferrarini G, Pagliarello I, Sartoris S, Ugel S, Marigo I, Molon B, Bronte V (2014) The emerging immunological role of post-translational modifications by reactive nitrogen species in cancer microenvironment. Front Immunol 5:69
Jacquelot N, Yamazaki T, Roberti MP, Duong CPM, Andrews MC, Verlingue L, Ferrere G, Becharef S, Vetizou M, Daillere R, Messaoudene M, Enot DP, Stoll G, Ugel S, Marigo I, Foong Ngiow S, Marabelle A, Prevost-Blondel A, Gaudreau PO, Gopalakrishnan V, Eggermont AM, Opolon P, Klein C, Madonna G, Ascierto PA, Sucker A, Schadendorf D, Smyth MJ, Soria JC, Kroemer G, Bronte V, Wargo J, Zitvogel L (2019) Sustained Type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res 29:846–861
Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AH, Ugel S, Sasso MS, Qualls JE, Kratochvill F, Zanovello P, Molon B, Ries CH, Runza V, Hoves S, Bilocq AM, Bindea G, Mazza EM, Bicciato S, Galon J, Murray PJ, Bronte V (2016) T cell cancer therapy requires CD40-CD40L activation of tumor necrosis factor and inducible nitric-oxide-synthase-producing dendritic cells. Cancer Cell 30:651
Daye D, Wellen KE (2012) Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol 23:362–369
Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G (2011) Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481:380–384
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105:18782–18787
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, Kimmelman AC (2013) Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496:101–105
Hammami I, Chen J, Murschel F, Bronte V, De Crescenzo G, Jolicoeur M (2012) Immunosuppressive activity enhances central carbon metabolism and bioenergetics in myeloid-derived suppressor cells in vitro models. BMC Cell Biol 13:18
Boutte AM, McDonald WH, Shyr Y, Yang L, Lin PC (2011) Characterization of the MDSC proteome associated with metastatic murine mammary tumors using label-free mass spectrometry and shotgun proteomics. PLoS ONE 6:e22446
Mehta K, Fok J, Miller FR, Koul D, Sahin AA (2004) Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res 10:8068–8076
Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL (1998) Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281:1191–1193
McGaha TL, Huang L, Lemos H, Metz R, Mautino M, Prendergast GC, Mellor AL (2012) Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev 249:135–157
Shinde R, McGaha TL (2018) The aryl hydrocarbon receptor: connecting immunity to the microenvironment. Trends Immunol 39:1005–1020
Smith BW, Rozelle SS, Leung A, Ubellacker J, Parks A, Nah SK, French D, Gadue P, Monti S, Chui DH, Steinberg MH, Frelinger AL, Michelson AD, Theberge R, McComb ME, Costello CE, Kotton DN, Mostoslavsky G, Sherr DH, Murphy GJ (2013) The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation. Blood 122:376–385
Yeste A, Takenaka MC, Mascanfroni ID, Nadeau M, Kenison JE, Patel B, Tukpah AM, Babon JA, DeNicola M, Kent SC, Pozo D, Quintana FJ (2016) Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci Signal 9:ra61
Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, Yin H, Zhou J (2013) Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol 43:2943–2955
Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W, Rodriguez PC, Ochoa AC (2015) Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res 3:1236–1247
Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC, Ochoa AC (2017) Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology 6:e1344804
Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, Ricciotti E, DiRusso C, Murphy ME, Vonderheide RH, Lieberman PM, Mulligan C, Nam B, Hockstein N, Masters G, Guarino M, Lin C, Nefedova Y, Black P, Kagan VE, Gabrilovich DI (2019) Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 569:73–78
Goffaux G, Hammami I, Jolicoeur M (2017) A dynamic metabolic flux analysis of myeloid-derived suppressor cells confirms immunosuppression-related metabolic plasticity. Sci Rep 7:9850
Patel S, Fu S, Mastio J, Dominguez GA, Purohit A, Kossenkov A, Lin C, Alicea-Torres K, Sehgal M, Nefedova Y, Zhou J, Languino LR, Clendenin C, Vonderheide RH, Mulligan C, Nam B, Hockstein N, Masters G, Guarino M, Schug ZT, Altieri DC, Gabrilovich DI (2018) Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol 19:1236–1247
LaGory EL, Giaccia AJ (2016) The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol 18:356–365
Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang X, Liu H, Lu Y, Liao J, Chen X, Chu Y (2014) SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1alpha-dependent glycolysis. Cancer Res 74:727–737
Baumann T, Dunkel A, Schmid C, Schmitt S, Hiltensperger M, Lohr K, Laketa V, Donakonda S, Ahting U, Lorenz-Depiereux B, Heil JE, Schredelseker J, Simeoni L, Fecher C, Korber N, Bauer T, Huser N, Hartmann D, Laschinger M, Eyerich K, Eyerich S, Anton M, Streeter M, Wang T, Schraven B, Spiegel D, Assaad F, Misgeld T, Zischka H, Murray PJ, Heine A, Heikenwalder M, Korn T, Dawid C, Hofmann T, Knolle PA, Hochst B (2020) Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal. Nat Immunol 21:555–566
Platten M, Nollen EAA, Rohrig UF, Fallarino F, Opitz CA (2019) Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov 18:379–401
Mondanelli G, Ugel S, Grohmann U, Bronte V (2017) The immune regulation in cancer by the amino acid metabolizing enzymes ARG and IDO. Curr Opin Pharmacol 35:30–39
Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, Arance A, Carlino MS, Grob JJ, Kim TM, Demidov L, Robert C, Larkin J, Anderson JR, Maleski J, Jones M, Diede SJ, Mitchell TC (2019) Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol 20:1083–1097
Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EM, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U (2011) Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 12:870–878
Orabona C, Pallotta MT, Volpi C, Fallarino F, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Grohmann U, Puccetti P (2008) SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis. Proc Natl Acad Sci U S A 105:20828–20833
Mondanelli G, Bianchi R, Pallotta MT, Orabona C, Albini E, Iacono A, Belladonna ML, Vacca C, Fallarino F, Macchiarulo A, Ugel S, Bronte V, Gevi F, Zolla L, Verhaar A, Peppelenbosch M, Mazza EMC, Bicciato S, Laouar Y, Santambrogio L, Puccetti P, Volpi C, Grohmann U (2017) A relay pathway between arginine and tryptophan metabolism confers immunosuppressive properties on dendritic cells. Immunity 46:233–244
Stockwell BR (2022) Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185:2401–2421
Fiore A, Zeitler L, Russier M, Gross A, Hiller MK, Parker JL, Stier L, Kocher T, Newstead S, Murray PJ (2022) Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell 82:920–32 e7
Kjeldsen JW, Lorentzen CL, Martinenaite E, Ellebaek E, Donia M, Holmstroem RB, Klausen TW, Madsen CO, Ahmed SM, Weis-Banke SE, Holmstrom MO, Hendel HW, Ehrnrooth E, Zocca MB, Pedersen AW, Andersen MH, Svane IM (2021) A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat Med 27:2212–2223
Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, Goswami S, Allison JP (2021) The next decade of immune checkpoint therapy. Cancer Discov 11:838–857
Bally AP, Lu P, Tang Y, Austin JW, Scharer CD, Ahmed R, Boss JM (2015) NF-kappaB regulates PD-1 expression in macrophages. J Immunol 194:4545–4554
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, Connolly AJ, Weissman IL (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545:495–499
Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A (2009) PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A 106:6303–6308
Hamaidia M, Gazon H, Hoyos C, Hoffmann GB, Louis R, Duysinx B, Willems L (2019) Inhibition of EZH2 methyltransferase decreases immunoediting of mesothelioma cells by autologous macrophages through a PD-1-dependent mechanism. JCI Insight 4(18):e128474. https://doi.org/10.1172/jci.insight.128474
Zhao Y, Harrison DL, Song Y, Ji J, Huang J, Hui E (2018) Antigen-presenting cell-intrinsic PD-1 neutralizes PD-L1 in cis to attenuate PD-1 signaling in T cells. Cell Rep 24:379–90 e6
Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, Senju S, Ihn H, Nishimura Y, Oshiumi H (2018) Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res 78:5011–5022
Long Y, Yu X, Chen R, Tong Y, Gong L (2022) Noncanonical PD-1/PD-L1 axis in relation to the efficacy of anti-PD therapy. Front Immunol 13:910704
Chen W, Wang J, Jia L, Liu J, Tian Y (2016) Attenuation of the programmed cell death-1 pathway increases the M1 polarization of macrophages induced by zymosan. Cell Death Dis 7:e2115
Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P, Yang J, He W, Chen H, Jiao Z, Li Y (2018) Tumor-derived exosomes induce PD1(+) macrophage population in human gastric cancer that promotes disease progression. Oncogenesis 7:41
Weber R, Fleming V, Hu X, Nagibin V, Groth C, Altevogt P, Utikal J, Umansky V (2018) Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front Immunol 9:1310
Yu GT, Bu LL, Zhao YY, Mao L, Deng WW, Wu TF, Zhang WF, Sun ZJ (2016) CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma. Oncoimmunology 5:e1151594
Anfray C, Ummarino A, Andon FT, Allavena P (2019) Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells 9(1):46. https://doi.org/10.3390/cells9010046
Katzenelenbogen Y, Sheban F, Yalin A, Yofe I, Svetlichnyy D, Jaitin DA, Bornstein C, Moshe A, Keren-Shaul H, Cohen M, Wang SY, Li B, David E, Salame TM, Weiner A, Amit I (2020) Coupled scRNA-Seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell 182:872–85 e19
Viitala M, Virtakoivu R, Tadayon S, Rannikko J, Jalkanen S, Hollmen M (2019) Immunotherapeutic blockade of macrophage clever-1 reactivates the CD8(+) T-cell response against immunosuppressive tumors. Clin Cancer Res 25:3289–3303
Xu W, Dong J, Zheng Y, Zhou J, Yuan Y, Ta HM, Miller HE, Olson M, Rajasekaran K, Ernstoff MS, Wang D, Malarkannan S, Wang L (2019) Immune-checkpoint protein VISTA regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol Res 7:1497–1510
Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, Zhang J, Song C, Zarr M, Zhou X, Han X, Archer KA, O’Neill T, Herbst RS, Boto AN, Sanmamed MF, Langermann S, Rimm DL, Chen L (2019) Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med 25:656–666
Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, Loginicheva E, Gilfillan S, Cella M, Virgin HW, Unanue ER, Wang Y, Artyomov MN, Holtzman DM, Colonna M (2017) TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170:649–63 e13
Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB (2010) TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal 3:ra38
Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, Keren-Shaul H, David E, Zmora N, Eldar SM, Lubezky N, Shibolet O, Hill DA, Lazar MA, Colonna M, Ginhoux F, Shapiro H, Elinav E, Amit I (2019) Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178:686–98 e14
Molgora M, Esaulova E, Vermi W, Hou J, Chen Y, Luo J, Brioschi S, Bugatti M, Omodei AS, Ricci B, Fronick C, Panda SK, Takeuchi Y, Gubin MM, Faccio R, Cella M, Gilfillan S, Unanue ER, Artyomov MN, Schreiber RD, Colonna M (2020) TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell 182:886-900 e17
Ostendorf BN, Bilanovic J, Adaku N, Tafreshian KN, Tavora B, Vaughan RD, Tavazoie SF (2020) Common germline variants of the human APOE gene modulate melanoma progression and survival. Nat Med 26:1048–1053
Yu X, Feng B, He P, Shan L (2017) From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu Rev Phytopathol 55:109–137
Carbone C, Piro G, Agostini A, Delfino P, De Sanctis F, Nasca V, Spallotta F, Sette C, Martini M, Ugel S, Corbo V, Cappello P, Bria E, Scarpa A, Tortora G (2021) Intratumoral injection of TLR9 agonist promotes an immunopermissive microenvironment transition and causes cooperative antitumor activity in combination with anti-PD1 in pancreatic cancer. J Immunother Cancer 9(9):e002876. https://doi.org/10.1136/jitc-2021-002876
Shekarian T, Valsesia-Wittmann S, Brody J, Michallet MC, Depil S, Caux C, Marabelle A (2019) Pattern recognition receptors: immune targets to enhance cancer immunotherapy. Ann Oncol 30:2017
Sun L, Kees T, Almeida AS, Liu B, He XY, Ng D, Han X, Spector DL, McNeish IA, Gimotty P, Adams S, Egeblad M (2021) Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell 39:1361–74 e9
Jing W, McAllister D, Vonderhaar EP, Palen K, Riese MJ, Gershan J, Johnson BD, Dwinell MB (2019) STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer 7:115
Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, Coffman RL, Guiducci C (2016) Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci U S A 113:E7240–E7249
Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, Varner JA, Pu M, Messer KS, Guiducci C, Coffman RL, Kitaura K, Matsutani T, Suzuki R, Carson DA, Hayashi T, Cohen EE (2017) Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2(18):e93397. https://doi.org/10.1172/jci.insight.93397
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331:1612–1616
Byrne KT, Vonderheide RH (2016) CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep 15:2719–2732
O’Hara MH, O’Reilly EM, Varadhachary G, Wolff RA, Wainberg ZA, Ko AH, Fisher G, Rahma O, Lyman JP, Cabanski CR, Mick R, Gherardini PF, Kitch LJ, Xu J, Samuel T, Karakunnel J, Fairchild J, Bucktrout S, LaVallee TM, Selinsky C, Till JE, Carpenter EL, Alanio C, Byrne KT, Chen RO, Trifan OC, Dugan U, Horak C, Hubbard-Lucey VM, Wherry EJ, Ibrahim R, Vonderheide RH (2021) CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol 22:118–131
Chochi K, Ichikura T, Kinoshita M, Majima T, Shinomiya N, Tsujimoto H, Kawabata T, Sugasawa H, Ono S, Seki S, Mochizuki H (2008) Helicobacter pylori augments growth of gastric cancers via the lipopolysaccharide-toll-like receptor 4 pathway whereas its lipopolysaccharide attenuates antitumor activities of human mononuclear cells. Clin Cancer Res 14:2909–2917
Huang B, Zhao J, Shen S, Li H, He KL, Shen GX, Mayer L, Unkeless J, Li D, Yuan Y, Zhang GM, Xiong H, Feng ZH (2007) Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res 67:4346–4352
Schmid MC, Khan SQ, Kaneda MM, Pathria P, Shepard R, Louis TL, Anand S, Woo G, Leem C, Faridi MH, Geraghty T, Rajagopalan A, Gupta S, Ahmed M, Vazquez-Padron RI, Cheresh DA, Gupta V, Varner JA (2018) Integrin CD11b activation drives anti-tumor innate immunity. Nat Commun 9:5379
Sharma MD, Rodriguez PC, Koehn BH, Baban B, Cui Y, Guo G, Shimoda M, Pacholczyk R, Shi H, Lee EJ, Xu H, Johnson TS, He Y, Mergoub T, Venable C, Bronte V, Wolchok JD, Blazar BR, Munn DH (2018) Activation of p53 in immature myeloid precursor cells controls differentiation into Ly6c(+)CD103(+) monocytic antigen-presenting cells in tumors. Immunity 48:91-106 e6
Kaczanowska S, Beury DW, Gopalan V, Tycko AK, Qin H, Clements ME, Drake J, Nwanze C, Murgai M, Rae Z, Ju W, Alexander KA, Kline J, Contreras CF, Wessel KM, Patel S, Hannenhalli S, Kelly MC, Kaplan RN (2021) Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell 184:2033–52 e21
Shields CWt, Evans MA, Wang LL, Baugh N, Iyer S, Wu D, Zhao Z, Pusuluri A, Ukidve A, Pan DC, Mitragotri S (2020) Cellular backpacks for macrophage immunotherapy. Sci Adv 6:eaaz6579
Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, Cummins KD, Shen F, Shan X, Veliz K, Blouch K, Yashiro-Ohtani Y, Kenderian SS, Kim MY, O’Connor RS, Wallace SR, Kozlowski MS, Marchione DM, Shestov M, Garcia BA, June CH, Gill S (2020) Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol 38:947–953
Shi J, Kantoff PW, Wooster R, Farokhzad OC (2017) Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 17:20–37
Amoozgar Z, Goldberg MS (2015) Targeting myeloid cells using nanoparticles to improve cancer immunotherapy. Adv Drug Deliv Rev 91:38–51
Lee NK, Kim SN, Park CG (2021) Immune cell targeting nanoparticles: a review. Biomater Res 25:44
Zhu S, Niu M, O’Mary H, Cui Z (2013) Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Mol Pharm 10:3525–3530
Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC, Stephan MT (2019) Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun 10:3974
Wang Y, Lin YX, Qiao SL, An HW, Ma Y, Qiao ZY, Rajapaksha RP, Wang H (2017) Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials 112:153–163
Zilio S, Vella JL, De la Fuente AC, Daftarian PM, Weed DT, Kaifer A, Marigo I, Leone K, Bronte V, Serafini P (2017) 4PD functionalized dendrimers: a flexible tool for in vivo gene silencing of tumor-educated myeloid cells. J Immunol 198:4166–4177
Acknowledgements
We thank Prof. Vincenzo Bronte and all components of the Immunology Section at the University of Verona (Bronte lab; #Immunologia1) for providing feedback on this manuscript and in particular Davide Rizzini for the artwork. We would like to thank Biorender for providing a platform for creative artwork.
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. This work was supported by the PRIN programs of the Italian Ministry of Education, University and Research (MIUR, PI: Ugel S.; CUP: B38D19000140006, PI: De Sanctis F.; CUP: B39J22001200001); Associazione Italiana per la Ricerca sul Cancro (AIRC, PI: Ugel S.; Project: 21509); and Fondazione Cariverona (PI: Ugel S.; Enact project).
Author information
Authors and Affiliations
Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francesco De Sanctis and Annalisa Adamo shared first authors.
This article is a contribution to the special issue on: Novel immunotherapeutic combinations moving forward: The modulation of the immunosuppressive microenvironment - Guest Editor: Mads Hald Andersen
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Sanctis, F., Adamo, A., Canè, S. et al. Targeting tumour-reprogrammed myeloid cells: the new battleground in cancer immunotherapy. Semin Immunopathol 45, 163–186 (2023). https://doi.org/10.1007/s00281-022-00965-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-022-00965-1