Abstract
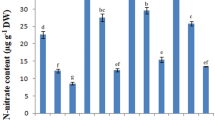
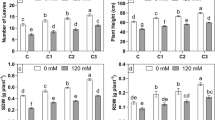
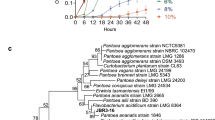
Plant growth and production are adversely affected by soil salinity. A plant growth-promoting bacteria (PGPB) designated as the “I4 strain” of Bacillus mojavensis was isolated from Tunisian soil (Sfax, Tunisia) and showed the ability to be grown in the presence of NaCl concentrations ranging from 0 to 10% in Luria Bertani (LB) medium. The PGPB-mediated salt tolerance in durum wheat was evaluated. The physiological parameters such as growth, shoot and root length, dry and fresh weight were higher in I4-inoculated wheat plants in comparison with non-treated plants under salt stress. Results showed that this strain promoted wheat growth and preserved the membrane damage by notably lowering the electrolytes leakage and malondialdehyde content in contrast to non-inoculated plants. Moreover, leaf chlorophyll content, biochemical parameters and antioxidant enzyme activities measurement showed a better salt and heavy metal stress adaptation of the I4-inoculated plants. Due to these outcomes, it could be suggested that the inoculation of the PGPB I4 strain enhanced the wheat plant’s growth, especially under salt stress conditions. This study confirms the ameliorative role played by PGPB in tolerating salt stress in wheat and their potential use as biofertilizers to enhance its growth in saline soil and help in promoting this plant’s culture to provide food security under these perturbed global circumstances.




Similar content being viewed by others
Data Availability
Not applicable.
References
Chaudhry S, Sidhu GPS (2022) Climate change regulated abiotic stress mechanisms in plants: a comprehensive review. Plant Cell Rep 41(1):1–31. https://doi.org/10.1007/s00299-021-02759-5
Etesami H, Maheshwari DK (2018) Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: action mechanisms and future prospects. Ecotoxicol Environ Saf 156:225–246. https://doi.org/10.1016/j.ecoenv.2018.03.013
Numan M, Bashir S, Khan Y, Mumtaz R, Shinwari ZK, Khan AL et al (2018) Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol Res 209:21–32. https://doi.org/10.1016/j.micres.2018.02.003
Charfeddine S, Charfeddine M, Hanana M, Gargouri-Bouzid R (2019) Ectopic expression of a grape vine vacuolar NHX antiporter enhances transgenic potato plant tolerance to salinity. J Plant Biochem Biotechnol 28(1):50–62. https://doi.org/10.1007/s13562-018-0462-x
Singh RP, Jha P, Jha PN (2015) The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J Plant Physiol 184:57–67. https://doi.org/10.1016/j.jplph.2015.07.002
Chandra P, Wunnava A, Verma P, Chandra A, Sharma RK (2021) Strategies to mitigate the adverse effect of drought stress on crop plants—influences of soil bacteria: a review. Pedosphere 31(3):496–509. https://doi.org/10.1016/S1002-0160(20)60092-3
Ghazala I, Haddar A, Romdhane M, Ellouz-Chaanouni S (2016) Screening and molecular identification of new microbial strains for production of enzymes of biotechnological interest. Braz Arch Biol Technol. https://doi.org/10.1590/1678-4324-2016150152
Ghazala I, Bouassida M, Krichen F, Benito J, Chaabouni Ellouz S, Haddar A (2017) Anionic lipopeptides from Bacillus mojavensis I4 as effective antihypertensive agents: production, characterization and identification. Eng Life Sci. https://doi.org/10.1002/elsc.201700020
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Qingping H, Jianguo X (2011) A simple double-layered chrome azurol S agar (SD- CASA) plate assay to optimize the production of siderophores by a potential biocontrol agent Bacillus. Afr J Microbiol Res 5:4321–4327
Payne SM (1994) Detection, isolation, and characterization of siderophores. Methods Enzymol 235:329–344. https://doi.org/10.1016/0076-6879(94)35151-1
Acuña JJ, Jorquera MA, Martínez OA, Menezes-Blackburn D, Fernández MT, Marschner P et al (2011) Indole acetic acid and phytase activity produced by rhizosphere bacilli as affected by pH and metals. J Soil Sci Plant Nutr 11:1–12
Sgroy V, Cassan F, Masciarelli O, Del Papa MF, Lagares A, Luna V (2009) Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl Microbiol Biotechnol 85(2):371–381. https://doi.org/10.1007/s00253-009-2116-3
Ngamau CN, Matiru VN, Tani A, Muthuri CW (2012) Isolation and identification of endophytic bacteria of bananas (Musa spp.) in Kenya and their potential as biofertilizers for sustainable banana production. Afr J Microbiol Res 6:6414–6422
Mefteh FB, Daoud A, Chenari Bouket A, Alenezi FN, Luptakova L, Rateb ME et al (2017) Fungal root microbiome from healthy and brittle leaf diseased date palm trees (Phoenix dactylifera L.) Reveals a hidden untapped arsenal of antibacterial and broad spectrum antifungal secondary metabolites. Front Microbiol 8:307. https://doi.org/10.3389/fmicb.2017.00307
Kloepper JW, Rodríguez-Kábana R, McInroy JA, Collins DJ (1991) Analysis of populations and physiological characterization of microorganisms in rhizospheres of plants with antagonistic properties to phytopathogenic nematodes. Plant Soil 136(1):95–102. https://doi.org/10.1007/BF02465224
El Arbi A, Rochex A, Chataigné G, Béchet M, Lecouturier D, Arnauld S et al (2016) The Tunisian oasis ecosystem is a source of antagonistic Bacillus spp. producing diverse antifungal lipopeptides. Res Microbiol 167(1):46–57. https://doi.org/10.1016/j.resmic.2015.09.003
Ansari F, Ahmad I (2018) Plant growth promoting attributes and alleviation of salt stress to wheat by biofilm forming Brevibacterium sp. FAB3 isolated from rhizospheric soil. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2018.08.003
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1–15. https://doi.org/10.1104/pp.24.1.1
Shukla PS, Agarwal PK, Jha B (2012) Improved salinity tolerance of Arachishypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J Plant Growth Regul 31(2):195–206. https://doi.org/10.1007/s00344-011-9231-y
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Analytical Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Hernández JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115(2):251–257. https://doi.org/10.1034/j.1399-3054.2002.1150211.x
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127(4):1781–1787. https://doi.org/10.1104/pp.010497
Sairam RK, Srivastava GC (2002) Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162(6):897–904. https://doi.org/10.1016/S0168-9452(02)00037-7
Rodriguez AA, Ramiro Lascano H, Bustos D, Taleisnik E (2007) Salinity-induced decrease in NADPH oxidase activity in the maize leaf blade elongation zone. J Plant Physiol 164(3):223–230. https://doi.org/10.1016/j.jplph.2006.07.014
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P (2021) Regulation of plant responses to salt stress. Int J Mol Sci. https://doi.org/10.3390/ijms22094609
Souri MK, Tohidloo G (2019) Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem Biol Technol Agric 6(1):26. https://doi.org/10.1186/s40538-019-0169-9
Fattahi M, Mohammadkhani A, Shiran B, Baninasab B, Ravash R, Gogorcena Y (2021) Beneficial effect of mycorrhiza on nutritional uptake and oxidative balance in pistachio (Pistacia spp.) rootstocks submitted to drought and salinity stress. Sci Horticul 281:109937. https://doi.org/10.1016/j.scienta.2021.109937
Hnilickova H, Kraus K, Vachova P, Hnilicka F (2021) Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants. https://doi.org/10.3390/plants10050845
Souri M, Naiji M (2018) Nutritional value and mineral concentrations of sweet basil under organic compared to chemical fertilization. Acta scientiarum Polonorum Hortorum cultus. https://doi.org/10.24326/asphc.2018.2.14
Gupta S, Pandey S (2019) ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in french bean (Phaseolus vulgaris) plants. Front Microbiol 10:1506. https://doi.org/10.3389/fmicb.2019.01506
Manasa M, Ravinder P, Gopalakrishnan S, Srinivas V, Sayyed RZ, El Enshasy HA et al (2021) Co-inoculation of Bacillus spp. for growth promotion and iron fortification in Sorghum. Sustainability. https://doi.org/10.3390/su132112091
Ben Khedher S, Mejdoub-Trabelsi B, Tounsi S (2021) Biological potential of Bacillus subtilis V26 for the control of Fusarium wilt and tuber dry rot on potato caused by Fusarium species and the promotion of plant growth. Biol Control 152:104444. https://doi.org/10.1016/j.biocontrol.2020.104444
Sandilya SP, Jeevan B, Subrahmanyam G, Dutta K, Vijay N, Bhattacharyya N et al (2022) Co-inoculation of native multi-trait plant growth promoting rhizobacteria promotes plant growth and suppresses Alternaria blight disease in castor (Ricinus communis L.). Heliyon 8(12):e11886. https://doi.org/10.1016/j.heliyon.2022.e11886
Ghosh S, Pal S, Chakraborty N (2015) The qualitative and quantitative assay of siderophore production by some microorganisms and effect of different media on its production. Int J Chem Sci 13:1621–1629
Egamberdieva D, Wirth S, Li L, Abd-Allah EF, Lindström K (2017) Microbial cooperation in the rhizosphere improves liquorice growth under salt stress. Bioengineered 8(4):433–438. https://doi.org/10.1080/21655979.2016.1250983
Oteino N, Lally RD, Kiwanuka S, Lloyd A, Ryan D, Germaine KJ et al (2015) Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol 6:745. https://doi.org/10.3389/fmicb.2015.00745
Etesami H, Alikhani HA (2017) Evaluation of Gram-positive rhizosphere and endophytic bacteria for biological control of fungal rice (Oryzia sativa L.) pathogens. Eur J Plant Pathol 147(1):7–14. https://doi.org/10.1007/s10658-016-0981-z
Orhan F (2016) Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz J Microbiol 47(3):621–627. https://doi.org/10.1016/j.bjm.2016.04.001
Lee DG, Lee JM, Choi CG, Lee H, Moon JC, Chung N (2021) Effect of plant growth-promoting rhizobacterial treatment on growth and physiological characteristics of Triticum aestivum L. under salt stress. Appl Biol Chem 64(1):89. https://doi.org/10.1186/s13765-021-00663-w
Alexander A, Singh VK, Mishra A (2020) Halotolerant PGPR Stenotrophomonas maltophilia BJ01 induces salt tolerance by modulating physiology and biochemical activities of Arachis hypogaea. Front Microbiol 11:568289. https://doi.org/10.3389/fmicb.2020.568289
Sapre S, Gontia-Mishra I, Tiwari S (2018) Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol Res 206:25–32. https://doi.org/10.1016/j.micres.2017.09.009
Wungrampha S, Joshi R, Singla-Pareek SL, Pareek A (2018) Photosynthesis and salinity: are these mutually exclusive? Photosynthetica 56(1):366–381. https://doi.org/10.1007/s11099-017-0763-7
Akbari A, Gharanjik S, Koobaz P, Sadeghi A (2020) Plant growth promoting Streptomyces strains are selectively interacting with the wheat cultivars especially in saline conditions. Heliyon 6(2):e03445. https://doi.org/10.1016/j.heliyon.2020.e03445
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167(3):527–533. https://doi.org/10.1016/j.plantsci.2004.04.020
Kasim WA, Osman ME, Omar MN, Abd El-Daim IA, Bejai S, Meijer J (2013) Control of drought stress in wheat using plant-growth-promoting bacteria. J Plant Growth Regul 32(1):122–130. https://doi.org/10.1007/s00344-012-9283-7
Manaf HH, Zayed MS (2015) Productivity of cowpea as affected by salt stress in presence of endomycorrhizae and Pseudomonas fluorescens. Ann Agric Sci 60(2):219–226. https://doi.org/10.1016/j.aoas.2015.10.013
Zhou ZS, Guo K, Elbaz AA, Yang ZM (2009) Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ Exp Bot 65(1):27–34. https://doi.org/10.1016/j.envexpbot.2008.06.001
Funding
This work was funded by the Ministry of Higher Education and Scientific Research-Tunisia.
Author information
Authors and Affiliations
Contributions
IG, NC and MNS conceived and designed the research. IG and NC conducted the experiments. IG, NC, MNS and RGB analyzed the data, contributed towards writing the manuscript, read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent of Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghazala, I., Chiab, N., Saidi, M.N. et al. The Plant Growth-Promoting Bacteria Strain Bacillus mojavensis I4 Enhanced Salt Stress Tolerance in Durum Wheat. Curr Microbiol 80, 178 (2023). https://doi.org/10.1007/s00284-023-03288-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03288-y