Abstract
Multisystem inflammatory syndrome (MIS-C) is a pediatric hyperinflammation disorder caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It has now been reported from several countries the world over. Some of the clinical manifestations of MIS-C mimic Kawasaki disease (KD) shock syndrome. MIS-C develops 4–6 weeks following SARS-CoV-2 infection, and is presumably initiated by adaptive immune response. Though it has multisystem involvement, it is the cardiovascular manifestations that are most prominent. High titres of anti-SARS-CoV-2 antibodies are seen in these patients. As this is a new disease entity, its immunopathogenesis is not fully elucidated. Whether it has some overlap with KD is still unclear. Current treatment guidelines recommend use of intravenous immunoglobulin and high-dose corticosteroids as first-line treatment. Mortality rates of MIS-C are lower compared to adult forms of severe COVID-19 disease.
Similar content being viewed by others
Background
Kawasaki disease (KD) is a medium vessel vasculitis of undetermined etiology usually affecting children below 5 years [1,2,3]. Rowley et al. had hypothesized as far back as 2004 that an unidentified respiratory infectious agent with tropism to vascular tissue, likely a virus, could be linked to the etiology of KD [4]. This putative RNA virus, presumably a ubiquitous one, resulted in persistent infection in bronchial epithelium and macrophages and was associated with intracytoplasmic inclusions [5, 6].
The first report of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emanated from Wuhan, China in November 2019. It then spread rapidly around the world. In April 2020, Verdoni et al. reported a 30 times increase in incidence of KD from Bergamo, Italy since the onset of this pandemic [7]. Further, the authors also noted increased disease severity in patients with KD during this period. There has also been a noticeable increase in incidence of ‘Kawasaki-like illness’ in association with Coronavirus disease 2019 (COVID-19) pandemic [7,8,9,10,11]. Confirmation of infection with COVID-19 in these reports has been through serology and/or RT-PCR.
Exposure of children to SARS-CoV-2 has been reported to result in development of Multisystem Inflammatory Syndrome (MIS-C) in some of them. This syndrome mimics KD [12, 13]. Several terminologies have been used to describe this condition. These include Kawasaki-like syndrome (KLS), atypical Kawasaki disease, incomplete Kawasaki disease, SARS-CoV-2-induced Kawasaki-like Hyper-inflammatory Syndrome (SCiKH Syndrome) and Kawa-COVID-19 [8, 14]. While the Centers for Disease Control and Prevention (CDC), United States (US) has termed this presentation as MIS-C, the World Health Organization and the Royal College of Pediatrics and Child Health have used other terminologies (Table 1) [15,16,17]. In this review, we shall refer to this condition as MIS-C.
MIS-C and KD, however, differ in several clinical features. Gastrointestinal complications, shock and coagulopathy are more common in patients with MIS-C, but are unusual in classic KD. Classic KD is common in North East Asian countries, whereas MIS-C has been reported more commonly in patients of African, Hispanic or Latino ethnicity. KD is common in children below 5 years, whereas MIS-C is more common in older children [15].
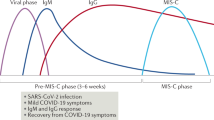
However, it is unclear whether the immunological mechanisms behind hyperinflammation of MIS-C are the same as that in adults with COVID-19. Cytokine storm induced hyperinflammation in adult COVID-19 is usually seen within 2 weeks, whereas MIS-C has been more commonly reported after 2 weeks of SARS-CoV-2 infection.
In view of different terminologies and varying descriptions, there is considerable confusion amongst clinicians regarding these syndromes. In this narrative review, we discuss the COVID-19-related syndromes that have emerged, especially in children.
Methods
In this review, we discuss various aspects of human coronavirus infections in children including epidemiology and the immuno-pathological mechanisms underlying MIS-C. We have also compared immune responses to SARS-CoV-2 in children with that in adults.
Search strategy
Objective of this narrative review is to discuss the immunological mechanisms, clinical features and treatment of severe COVID-19 disease and MIS-C [18]. A literature search through Medline/Pubmed, Scopus and Embase on COVID-19 disease in children, multisystem inflammatory syndrome in children and Kawasaki disease was carried out for the period 01 December, 2019—31 August, 2020. We have used following key words for the literature search: ‘Coronavirus disease-19’, ‘COVID-19’, ‘human coronaviruses’, ‘children’, ‘severe acute respiratory syndrome coronavirus-2’, ‘SARS-CoV-2’, ‘Kawasaki disease’, ‘Kawasaki disease shock syndrome’, ‘Kawasaki shock syndrome’, “Kawasaki-like disease”, and ‘Kawasaki like syndrome’, ‘SARS-CoV-2-induced Kawasaki-like Hyper-inflammatory Syndrome’, ‘SCiKH Syndrome’, ‘Kawa-COVID-19’, ‘Multisystem Inflammatory Syndrome’, ‘MIS-C’, ‘Multisystem inflammatory disorder in children and adolescents’, ‘Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2’, and ‘PIMS-TS’. We included original studies, reviews, view points, commentaries, case series and case reports which were relevant to our objectives. Reports published in languages other than English were excluded (Supplementary figure-1). Finally, the information was synthesized in a logical sequence with expert inputs from the senior authors.
Epidemiology of human coronaviruses in children
Human coronavirus (HCoV) in children with respiratory infections
Young children (especially those below the age of 3 years) as well as older individuals (above the age of 50 years) are considered to be high-risk groups for HCoV infections. Several studies have identified HCoV in respiratory tract specimens in children. Esper et al. reported that 8.8% of children hospitalized in the United States with respiratory infections were positive for HCoV [19]. Dijkman et al. have reported that HCoV was positive in 14% of hospitalized children aged below 2 years [20]. Majority of these infections were due to HCoV-OC43 and HCoV-NL63 strains. Similarly, seroprevalence data have also suggested that HCoV-OC43 and HCoV-NL63 are more common in infants [20]. It has also been suggested that infection by one HCoV strain may confer protection against other coronaviruses [20]. However, studies from China, Hong Kong, Australia, Brazil and Greece reported seroprevalence rates of HCoV to be less than 5% amongst children with upper respiratory tract infections [21,22,23,24]. In addition, it was also noted that influenza virus is the most common co-infection of HCoV [21]. Overall, however, seasonal HCoV may be responsible for 5–14% of respiratory infections in children.
COVID-19
Coronavirus disease (COVID-19) is an infectious disease caused by a newly discovered coronavirus SARS-CoV-2, which is a member of Betacoronavirus family. SARS-CoV-2 infection is largely asymptomatic in most individuals. Some patients may develop mild-to-moderate respiratory symptoms. A minority of patients develop severe disease. This is more likely in elderly patients and in patients with co-morbidities such as diabetes and cardiovascular disease. Transmission of SARS-CoV-2 occurs through inhalation of respiratory droplets from an infected person and/or contact with surfaces contaminated with virus. It has been shown that transmissibility of SARS-CoV-2 is higher when compared to other coronaviruses such as SARS-CoV (2002) and Middle East Respiratory Syndrome (MERS).
Phenotypes of COVID-19 infection observed in children and differences from adult disease
SARS-CoV-2 enters the host cell through binding of its spike (S) protein to the ACE2 receptor. This entry is facilitated by priming of S-protein through proteases (e.g., TMPRSS2) [25]. Recent studies have shown that adults have higher expression of ACE2 and TMPRSS2 on alveolar lining cells as compared to children [26]. This explains why children have a lower propensity to develop respiratory complications after SARS-CoV-2.
During early days of COVID-19 pandemic, it was suggested that children are not susceptible to COVID‐19 infection. During the course of the ongoing pandemic, however, it has become clear that children can also get COVID-19 infection albeit much less frequently.
Pediatric COVID-19 typically presents with mild symptoms such as cough, fever, sore throat and diarrhea. Lower respiratory tract symptoms are usually less prominent in children as compared to adults. Majority of children with symptomatic COVID-19 have mild disease and progression to acute respiratory distress syndrome (ARDS), which is a hallmark of adult COVID-19 disease, is even less common. Mortality associated with COVID-19 is also much lower in children as compared to adults (< 0.1% versus 5–15%). Laboratory manifestations include leucopenia, lymphopenia, mild elevation in transaminases and elevated inflammatory markers (i.e., C-reactive protein and procalcitonin). Computed tomography chest findings in children with severe COVID-19 disease (such as ground glass opacities, consolidation) are similar to adult COVID-19 disease (Tables 2, 3).
Although COVID-19 in children is usually mild, a rare novel post-COVID syndrome known as MIS-C has emerged in children and adolescents (Table 1). This shows close similarity to other hyper-inflammatory syndromes in children such as KD shock syndrome, toxic shock syndrome and macrophage activation syndrome [27] (Table 3).
Clinical correlation of immune response in pediatric COVID-19
Whether the milder clinical manifestations after SARS-CoV-2 infection in children as compared to adults can be ascribed to a more robust humoral immune response in the former remains conjectural.
Children with mild COVID infection have been reported to have increased number of IgG producing B cells as well as lower levels of acute phase reactants such as C-reactive protein (CRP) and IL-6 [28, 29]. Lymphocytopenia, on the other hand, is more common in adult patients with severe COVID-19 infection as compared to children [29,30,31]. Children with pneumonia in the setting of COVID-19 have low levels of serum IgA and regulatory (CD4 + CD25 +) T cells, increased levels of high sensitivity (hs)-CRP, IL-10 and procalcitonin [32]. This may, therefore, suggest that dampened serum IgA and decreased circulating regulatory T cells contribute to the deranged immune response against SARS-CoV-2.
SARS-CoV-2 infection also causes chilblains, also known as ‘COVID toes’. This has been reported in children and young adults infected with COVID-19. It probably results from vascular damage and necrosis of SARS-CoV-2-infected endothelial cells resulting in ischemia. Inclusion viral particles were also noted in endothelial cells on electron microscopy in these patients, in addition to dense lymphocytic infiltration with CD4 and CD8 T cells as well as B cells [33]. IgA, IgM and C3 deposits too have been noted in some cases [34, 35]. Antinuclear antibodies are positive in some patients as well [36]. Pathological lesions seen in ‘COVID toes’ mimic those of autoimmune-related chilblains [33, 34].
In summary, children with COVID-19 tend to have an appropriate early innate and humoral immune response to HCoV infections to clear the virus, followed by a less intense late immune response in majority. This is in contrast to an early-impaired immune response, followed by a late severe immune-mediated inflammation in COVID-19 disease of adults.
Treatment of pediatric COVID-19
Treatment of COVID-19 is largely supportive. Patients with mild-to-moderate symptoms can be managed with home isolation. Supportive therapy includes maintenance of adequate hydration, appropriate calorie intake and psychosocial support. For fever, paracetamol is recommended. Patients having severe symptoms require hospitalization and intensive care. In addition to standard supportive therapy (i.e., oxygen supplementation, maintenance of fluid and electrolyte balance and cardiovascular support), these patients are usually put on broad-spectrum antimicrobials and antivirals (e.g., remdesivir and lopinavir/ritonavir). Few patients with severe COVID-19 disease and ARDS may require immunomodulatory therapies for the putative hyper-inflammatory state. These therapies include corticosteroids and biologics such as tocilizumab and anakinra.
MIS-C
Definitions of this have been listed in Table 1.
Clinical features of multisystem inflammatory syndrome in children (MIS-C)
Although initial data suggested that COVID-19 causes mild disease in children [37, 38], several centers in Europe and United States had identified a new hyper-inflammatory syndrome (HIS) associated with this infection [7, 11, 39, 40]. Many case definitions have been proposed for this condition [16, 17, 41]. However, the features that are common to all include the presence of fever, hyper-inflammatory state and organ dysfunction (Table 1).
Fever is a universal feature in patients with MIS-C. Other associated clinical findings include cutaneous manifestations, abdominal symptoms and cardiovascular collapse. Typically, this entity is seen in older children (> 5 years) and median age of patients in various studies has ranged from 7.5 to 10 years [7, 8, 11, 39, 42, 43]. Unlike KD, there is no significant male predominance. Hemodynamic instability is present in 60–80% patients [8, 11, 40, 42]. Gastrointestinal manifestations are very common and include abdominal pain, diarrhea and vomiting. Some patients have undergone exploratory laparotomy. Neurological features (e.g., headache, meningeal signs and altered sensorium) are also common [7, 8, 42].
One of the largest cohorts [n = 186; median age 8.3 years; 115 (62%) boys] of this entity has been reported from United States (US) [11]. Fever was the predominant complaint with 90% patients having been febrile for ≥ 4 days. Systemic features included gastrointestinal (92%), cardiovascular (80%), hematological (76%), mucocutaneous (74%) and respiratory involvement (70%). Approximately 50% required vasoactive support. Majority of patients (71%) in this cohort had involvement of four or more organ systems. Patients having features consistent with KD were usually below 5.
Whittaker et al. have recently published a large experience from the United Kingdom (UK) [42]. They reported 58 patients with PIMS-TS and identified three different types of clinical presentations:
-
I.
Persistent fever and elevated inflammatory markers:
-
These patients did not have features of organ dysfunction, KD or toxic shock syndrome (TSS)
-
-
II.
Fever along with cardiovascular collapse and elevated cardiac biomarkers:
-
These patients had predominant cardiac manifestations including left ventricular dysfunction and arrhythmias
-
Cardiac troponin and pro-BNP were significantly elevated in these patients
-
-
III.
Patients presenting as KD or KD shock syndrome (KDSS):
-
These patients fulfilled the American Heart Association diagnostic criteria for KD
-
It has been seen that patients with MIS-C may progress to single- or multiorgan dysfunction due to persistent fever and hyperinflammation [8, 11, 44]. Feldstein et al. have shown that 80% patients required intensive care, 20% required mechanical ventilation and 4% received extracorporeal membrane oxygenation (ECMO) [11]. Due to the ongoing hyper-inflammatory state, chances of development of macrophage activation syndrome (MAS) are high in these patients [8, 45].
Cardiovascular complications
Cardiovascular complications are the most prominent manifestations in patients with MIS-C. Cardiac biomarkers including NT-pro-BNP and troponin levels are extremely high compared to historical KD cohorts and indicate heart failure and myocardial damage in MIS-C. Symptomatic myocarditis has been reported in 40–80% of patients with MIS-C [7, 8, 11, 40]. In contrast, symptomatic myocarditis is seen in < 5% of patients with KD [46,47,48]. Pouletty et al. reported that severe disease requiring intensive care due to myocarditis was seen in approximately 50% of patients with MIS-C and the risk was higher in older children [8].
Coronary artery abnormalities (CAAs) have been reported in 9–24% of patients with MIS-C [8, 11, 42, 43, 45, 49, 50]. CAAs are in form of dilatation or small-sized aneurysms in most patients. Pericarditis, pericardial effusion and valvular regurgitation have also been reported [8, 11, 51]. Electrocardiographic abnormalities include prolonged PR interval, T wave and ST segment changes.
Differential diagnosis
Diagnosis of MIS-C should be considered in children presenting with unexplained high-grade fever lasting more than 4 days [11]. A high degree of suspicion is essential as patients with MIS-C may deteriorate rapidly and develop cardiovascular collapse. In view of multisystem involvement, organ dysfunction should be anticipated. Although MIS-C shares several similarities with KD, there are important differences as well. MIS-C can also closely mimic clinical features of KDSS, toxic shock syndrome and severe COVID-19 disease. These have been highlighted in Table 3.
Laboratory features
Baseline laboratory workup should include complete blood counts, liver function tests, renal functions tests, and an assay of inflammatory markers. A possibility of MAS should be considered whenever there is rapid clinical deterioration and relevant investigations must be carried out for early identification of this entity. Majority of patients with MIS-C appear to have a hyper-inflammatory state that manifests as neutrophilic leukocytosis, raised erythrocyte sedimentation rates, hyponatremia, hypertriglyceridemia, elevated levels of CRP, procalcitonin, d-dimer and serum ferritin. Patients with MIS-C usually have lower platelet counts and higher ferritin levels as compared to patients with KD. While lymphopenia has been noted in patients with MIS-C, neutrophilic leukocytosis is the norm in KD [8, 11, 42, 43, 45, 51]. For identification of SARS-CoV-2, both serology and RT-PCR should be performed [11, 42, 51,52,53]. Sample for serology should be taken before administration of intravenous immunoglobulin (IVIg) [52]. In the US cohort, approximately two-thirds of patients tested positive for SARS-CoV-2 infection either by RT-PCR or serology, or both. But the remaining one-third of patients who were negative on both tests had come in contact with COVID-19-positive individuals [11]. Rostad et al. have performed serological studies in children with MIS-C and compared the results with children who had usual COVID-19 infection, KD and hospital controls [54]. They showed that patients with MIS-C had higher levels of IgG SARS-CoV-2 receptor-binding domain. It appears that higher titers were associated with disease severity in children with MIS-C. It has been suggested that detailed infective workup and cultures should be performed for consideration of alternative diagnoses especially where laboratory evidence of current or past infection with SARS-CoV-2 is not forthcoming [8, 11, 42, 51].
Cardiac biomarkers, including NT-pro-BNP and troponins, should also be assayed. Both NT-pro-BNP and cardiac troponin levels are extremely high in patients with MIS-C compared to KD [8, 11, 42, 45, 55]. Whittaker et al. have shown that NT-pro-BNP levels were elevated in 83% patients, while troponins were increased in 68% patients [42]. 2D-echocardiography should be carried out for identification of myocarditis, pericarditis, valvular abnormalities and CAAs [48, 56, 57]. The electrocardiogram may show changes of myocardial strain. Blondiaux et al. carried out cardiac magnetic resonance imaging (CMRI) in four patients with MIS-C and myocarditis—three during acute phase and one during recovery phase [58]. CMRI finding were consistent with diffuse myocardial edema thereby suggesting that myocarditis in MIS-C is post-infectious in origin. Similar observations have also been made in patients with KD.
Treatment
In view of the novelty of the syndrome and similarity to KD, treatment regimens have been extrapolated from guidelines for management of patients with KD. Rapid and aggressive treatment options should be considered according to evolution of disease. Recently, the American College of Rheumatology (ACR) has published guidelines for treatment of MIS-C [59]. The ACR has recommended the use of intravenous immunoglobulin (IVIg) and/or high-dose corticosteroids as first-line therapy in these patients. Approximately 30–80% patients do not respond to IVIg alone and may require adjunctive immunomodulatory therapy to control inflammation [8, 11, 43, 49,50,51]. This is in contrast to classic KD where IVIg resistance has been seen in less than 15% patients [60]. Intravenous pulse methylprednisolone (10–30 mg/kg/day for 3–7 days followed by gradual tapering of oral prednisolone) has been found to be useful. Other therapeutic modalities that have been used in these patients include second dose of IVIg, anakinra, tocilizumab and infliximab [42, 43, 45, 61, 62].
Complications and outcome
-
1.
As patients with MIS-C have been reported to have propensity for multisystem involvement, the treating physician needs to be alert to development of myocarditis, MAS and renal impairment [8].
-
2.
MIS-C is a hyper-inflammatory state and can progress to MAS/cytokine storm syndrome. Pouletty et al. have compared patients with severe and non-severe forms of MIS-C [8]. They reported that higher age, and a serum ferritin > 1400 µg/L were the best discriminators for severe disease.
-
3.
Myocarditis can evolve rapidly and needs to be identified early. It usually responds to immunomodulatory therapy (i.e., IVIg and high-dose corticosteroids). Follow-up echocardiography at 6 weeks has shown normal left ventricular function in most patients [8, 11, 40].
-
4.
CAAs have also been reported in these patients. However, CAAs of MIS-C are usually in the form of ectasia or small dilatations [11, 40, 42, 50, 63].
-
5.
Mortality rate in the US cohort of MIS-C was 2% [11]. Overall mortality rates are lower compared to adults.
Kawasaki disease (KD), MIS-C and COV-HI in adults and pediatric COVID-19
KD and HCoV
Approximately 9% of patients with KD have recent history of respiratory infections (usually rhinovirus, adenovirus and influenza). Interval between onset of respiratory syndrome and development of KD is approximately 2 weeks [64, 65]. This subgroup of patients often has incomplete KD and is associated with high frequency of coronary aneurysms [66]. Huang et al. have shown that patients with KD and influenza co-infection had longer duration of fever and were more likely to be associated with delays in diagnosis [67].
Rowley et al. identified a ubiquitous RNA virus in the respiratory tract and hypothesized that this could be a causative agent for KD. Esper et al. have shown an association between KD and a previously unknown human coronavirus (HCoV) [68]. However, further studies could not establish a causal association with KD [69,70,71,72].
Immunological alterations in KD
KD has also been reported in adult patients with Human Immunodeficiency Virus infection as well as children with primary immunodeficiency disorders (PIDs) like X-linked agammaglobulinemia. Many of these patients (~ 50%) have incomplete forms of KD [73]. Genes involved in type I IFN have been found to be upregulated in patients with KD [74]. However, other studies have reported that expression of genes involved in type I IFN response were reduced in peripheral blood mononuclear cells of patients with KD [75,76,77]. It is likely that children with MIS-C may have an, as yet undeciphered, immune dysregulation state that is possibly triggered by SARS-CoV-2.
Stimulation of peripheral blood mononuclear cells (PBMCs) by Toll-like receptor (TLR)-9 ligand results in upregulation of IgA-antibody secreting cells (ASC) in patients with KD. On the other hand, IVIg treatment causes downregulation of IgA producing ASCs in KD [78, 79]. Higher expression of TLR-7, but not of TLR-9, is also noted in coronary arteries of patients with KD compared to controls [74].
Oligoclonal IgA responses have been demonstrated in vessel walls of patients with KD and this may be antigen driven [80, 81]. Inclusion bodies, resembling viral aggregates, have been shown to be present in bronchial epithelium and macrophages during acute phase of KD [5]. This shows that viral antigens can drive oligoclonal IgA responses in mucosal tissues and vascular tissues of patients with KD.
In an animal model of KD, IgA and C3 immune complexes were found to be deposited in cardiovascular tissues [73]. Deposition of immune complexes containing IgA, IgG, IgM and C3 in blood vessels has also been noted in a COVID-19 patient with vasculitis [82]. This suggests that both KD and MIS-C may represent some form of IgA vasculitis involving a lung–gut vascular axis and further that MIS-C may be triggered by SARS-CoV-2 infection.
The presence of IgA + plasma cells in arteries on conjunctival tissue has been noted in an adult patient with HIV infection complicated by KLS. Increased level of soluble tumor necrosis factor receptor (sTNFR) II was also seen in this patient [83]. Patients with KD and adult-onset viral KLS are known to have similar cytokine profiles with elevated levels of sTNFR II, CXCL11, CCL1 and CCL2 [84].
Immunological alterations in MIS-C, a severe pediatric COVID-19 disease
SARS-CoV-2 associated MIS-C usually appears a few weeks after onset of infection [8]. Toubiana et al. have shown that children with MIS-C presented 36–45 days after appearance of the first signs of COVID-19 or following contact with an individual who was either a confirmed or a presumed case of COVID-19 [43]. Therefore, there is a predictable delay between SARS-CoV-2 infection and KLS.
Higher number of mucosal homing T cells and higher expression of IL-17 were also seen in pediatric COVID-19 with MIS-C as compared to COVID-19 without MIS-C [76]. Diorio et al. recently reported the cytokine profiles and viral cycle thresholds of patients with usual severe COVID-19 and MIS-C in children [85]. These authors have shown that patients with severe COVID-19 disease presented with lower viral cycle threshold, whereas those with MIS-C had higher cycle threshold. Cytokine profile showed that levels of IL-10 and TNF-α were higher in MIS-C compared to severe COVID-19 without MIS-C [85]. This shows that development of MIS-C may be a post-infectious, immunologically mediated sequel of COVID-19. In addition, MIS-C has been reported more often in individuals with African–American and Latino ethnicities [42, 86].
Similarities between MIS-C and KD
Both KD and MIS-C are associated with a significant cytokine storm that results in systemic inflammation and may explain the myocardial dysfunction that is often seen in these patients [8, 40, 87]. In addition, elevated level of ferritin in these patients is a surrogate marker for MAS [88]. It has been noted that high levels of ferritin are associated with severe disease in SARS-CoV-2-associated MIS-C [8].
Mucosal biopsy of a patient with COVID-19 presenting with diarrhea and abdominal pain showed presence of SARS-CoV-2 in endothelial cells by immunochemistry. There was also evidence of small and medium vessel vasculitis, similar to that seen in patients with KD [89].
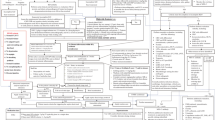
There have been some recent reports on detection of autoantibodies in patients with MIS-C. Target antigens for autoantibodies are expressed in mucosal and cardiac tissues, endothelial cells and cytokine molecules [90, 91]. These auto-antigens have also been reported in patients with KD [92]. Neutrophils and monocytes expressing Fcγ receptors are responsible for disease pathogenesis probably by engaging with autoantibodies and resulting in formation of immune complexes [90, 91]. IgG producing ASC have been known to increase during acute stage of KD and decrease after IVIg administration [79]. This again shows that autoantibodies or antibodies to SARS-CoV-2 may be contributing to disease pathogenesis in MIS-C and KD (Table 2 and Fig. 1). However, the precise pathogenetic mechanisms of MIS-C following SARS-CoV-2 infection still remain unclear.
Proposed immunological mechanism possibly triggered by antibody production in MIS-C. In genetically susceptible individuals, SARS-CoV-2 infections causes viral specific antibodies and there might be cross-reactive antibodies binding to host antigens. These antibodies may bind to Fcγ receptors in neutrophils and macrophages causing activation and secretion of pro-inflammatory cytokines that results in development of MIS-C. IFITM3 interferon-induced transmembrane protein-3, CD40LG cluster of differentiation 40 (CD40) ligand, HLA-B15:03 human leukocyte antigen (HLA) B15:03, ACE1 angiotensin-converting enzyme 1
Differences between MIS-C and COV-HI in adults
COVID-19-associated hyperinflammation (COV-HI) in adults is seen in patients with severe forms of disease. Hyperinflammation-induced cytokine storm in these patients predominantly involves the lungs resulting in ARDS, whereas MIS-C is a multiorgan cytokine storm that usually spares the lungs (Table 3). It is important to identify patients with COVID-19 having hyperinflammation, as this has management implications. Manson et al. have defined COV-HI when patients with COVID-19 have CRP values > 150 mg/L or when serum ferritin level is > 1500 µg/L [93]. Webb et al. have also proposed objective criteria for COVID-19 hyper-inflammatory syndrome (cHIS) based on fever, hematological dysfunction, macrophage activation, liver involvement, coagulation abnormalities and hypercytokinemia [94]. Patients with COV-HI require early escalation of respiratory support and have higher mortality [93, 94].
There have been several reports of Multisystem Inflammatory Syndrome in Adults (MIS-A), a condition that appears to be similar to MIS-C [95,96,97]. There is predominant involvement of cardiovascular and gastrointestinal systems [95,96,97,98]. Laboratory features include elevated inflammatory parameters (e.g., CRP and ferritin), raised d-dimer levels and lymphocytopenia [95,96,97].
Serological findings in MIS-C, KD and severe COVID-19
Approximately 70% of children with MIS-C have a positive antibody response against SARS-CoV-2. The RT-PCR test is positive in up to 60% [11, 86]. Following may be the reasons for low positivity rate of RT-PCR in patients with MIS-C:
-
1.
Interval between SARS-CoV-2 exposure and development of MIS-C varies from 2 to 4 weeks. During this time, virus may be cleared by neutralizing antibodies and immune cells. Therefore, MIS-C is predominantly a post-infectious inflammatory syndrome.
-
2.
Positivity rate of serology is reportedly higher than that of RT-PCR amongst those tested. In a recent French study on both asymptomatic and symptomatic patients, positivity of serology was 10.7%, while that of RT-PCR was 1.8% [99].
Patients who are seronegative, and also test negative on RT-PCR, usually have had contact with a COVID-19-positive individual [100]. This suggests that MIS-C is initiated by adaptive immune response (mediated by anti-SARS-COV-2 antibody or SARS-COV-2-induced de novo autoantibodies), unlike severe COVID-19 disease in absence of MIS-C that is driven predominantly by innate immune response (cytokine storm originating from neutrophils and macrophages) [90].
Conclusion
MIS-C is a hyper-inflammatory syndrome affecting multiple organs and is triggered by SARS-CoV-2 infection. It is usually seen 2–4 weeks following infection. Adaptive immune mechanisms have a major role to play in pathogenesis of this condition. Although clinical manifestations of MIS-C and KD may be overlapping, these appear to be two distinct clinical entities.
References
Jindal AK, Pilania RK, Prithvi A, Guleria S, Singh S (2019) Kawasaki disease: characteristics, diagnosis and unusual presentations. Expert Rev Clin Immunol
Singh S, Jindal AK, Pilania RK (2018) Diagnosis of Kawasaki disease. Int J Rheum Dis 21(1):36–44
Singh S, Vignesh P, Burgner D (2015) The epidemiology of Kawasaki disease: a global update. Arch Dis Child 100(11):1084–1088
Rowley AH, Baker SC, Shulman ST, Garcia FL, Guzman-Cottrill JA, Chou P et al (2004) Detection of antigen in bronchial epithelium and macrophages in acute Kawasaki disease by use of synthetic antibody. J Infect Dis 190(4):856–865
Rowley AH, Baker SC, Shulman ST, Fox LM, Takahashi K, Garcia FL et al (2005) Cytoplasmic inclusion bodies are detected by synthetic antibody in ciliated bronchial epithelium during acute Kawasaki disease. J Infect Dis 192(10):1757–1766
Rowley AH, Baker SC, Shulman ST, Garcia FL, Fox LM, Kos IM et al (2008) RNA-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PLoS ONE 3(2):e1582
Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M et al (2020) An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395(10239):1771–1778
Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N et al (2020) Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 79(8):999–1006
Ouldali N, Pouletty M, Mariani P, Beyler C, Blachier A, Bonacorsi S et al (2020) Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health 4(9):662–668
Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F et al (2020) SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill 25(22):2001010
Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF et al (2020) Multisystem inflammatory syndrome in US Children and adolescents. N Engl J Med 383(4):334–346
Abrams JY, Godfred-Cato SE, Oster ME, Chow EJ, Koumans EH, Bryant B et al (2020) Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr 226(45–54):e1
Akca UK, Kesici S, Ozsurekci Y, Aykan HH, Batu ED, Atalay E, et al (2020) Kawasaki-like disease in children with COVID-19. Rheumatol Int 40(12):2105–2115
Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S et al (2020) SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics 146(2):e20201711
CDC (2020) Multisystem Inflammatory Syndrome in Children (MIS-C) [Internet]. Centers for Disease Control and Prevention. https://www.cdc.gov/mis-c/hcp/. Accessed 11 Aug 2020
Multisystem inflammatory syndrome in children and adolescents with COVID-19 (2020) Scientific brief: World Health Organisation. https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-andadolescents-with-covid-19. Accessed 15 May 2020.
Royal College of Pediatrics and Child Health (2020) Guidance—Pediatric multisystem inflammatory syndrome temporally associated with COVID-19, 2020. https://www.rcpch.ac.uk/resources/guidance-pediatric-multisystem-inflammatory-syndrometemporally-associated-covid-19
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31(11):1409–1417
Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS (2005) Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis 191(4):492–498
Dijkman R, Jebbink MF, Gaunt E, Rossen JWA, Templeton KE, Kuijpers TW et al (2012) The dominance of human coronavirus OC43 and NL63 infections in infants. J Clin Virol 53(2):135–139
Zhang SF, Tuo JL, Huang XB, Zhu X, Zhang DM, Zhou K et al (2018) Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010–2015 in Guangzhou. PLoS ONE 13(1):e0191789
Lau SKP, Woo PCY, Yip CCY, Tse H, Tsoi H, Cheng VCC et al (2006) Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol 44(6):2063–2071
Chiu SS, Hung Chan K, Wing Chu K, Kwan SW, Guan Y, Man Poon LL et al (2005) Human Coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong. China Clin Infect Dis 40(12):1721–1729
Papa A, Papadimitriou E, de Souza Luna LK, Al Masri M, Souliou E, Eboriadou M et al (2007) Coronaviruses in children, Greece. Emerg Infect Dis 13(6):947–949
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271-280.e8
Muus C, Luecken MD, Eraslan G, Waghray A, Heimberg G, Sikkema L et al (2020) Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. BioRxiv 2020.04.19.049254
Han SB, Lee SY (2020) Macrophage activation syndrome in children with Kawasaki disease: diagnostic and therapeutic approaches. World J Pediatr. https://doi.org/10.1007/s12519-020-00360-6 (Epub ahead of print)
Zhang Y, Xu J, Jia R, Yi C, Gu W, Liu P et al (2020) Protective humoral immunity in SARS-CoV-2 infected pediatric patients. Cell Mol Immunol 17(7):768–770
Chen J, Zhang ZZ, Chen YK, Long QX, Tian WG, Deng HJ et al (2020) The clinical and immunological features of pediatric COVID-19 patients in China. Genes Dis. https://doi.org/10.1016/j.gendis.2020.03.008 (Epub ahead of print)
Henry BM, Benoit SW, de Oliveira MHS, Hsieh WC, Benoit J, Ballout RA et al (2020) Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): a pooled analysis and review. Clin Biochem 81:1–8
Wu H, Zhu H, Yuan C, Yao C, Luo W, Shen X et al (2020) Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open 3(6):e2010895
Li Y, Deng W, Xiong H, Li H, Chen Z, Nie Y et al (2020) Immune-related factors associated with pneumonia in 127 children with coronavirus disease 2019 in Wuhan. Pediatr Pulmonol. https://doi.org/10.1002/ppul.24907 (Epub ahead of print)
Colmenero I, Santonja C, Alonso-Riaño M, Noguera-Morel L, Hernández-Martín A, Andina D et al (2020) SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol 183(4):729–737
Kanitakis J, Lesort C, Danset M, Jullien D (2020) Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol 83(3):870–875
Rodríguez-Villa Lario A, Vega-Díez D, González-Cañete M, Gómez-Zubiaur A, Pérez-Mesonero R, Bandini M et al (2020) Histological findings in chilblain lupus-like COVID lesions: in search of an answer to understand their aetiology. J Eur Acad Dermatol Venereol 34(10):e572–e574
Herman A, Peeters C, Verroken A, Tromme I, Tennstedt D, Marot L et al (2020) Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol 156(9):998–1003
Meena J, Yadav J, Saini L, Yadav A, Kumar J (2020) Clinical features and outcome of SARS-CoV-2 infection in children: a systematic review and meta-analysis. Indian Pediatr 57(9):820–826
Liu W, Zhang Q, Chen J, Xiang R, Song H, Shu S et al (2020) Detection of Covid-19 in children in early January 2020 in Wuhan. China N Engl J Med 382(14):1370–1371
Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P (2020) Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395(10237):1607–1608
Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S et al (2020) Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.048360 (Epub ahead of print)
Centers for Disease Control and Prevention. Emergency preparedness and response: Health alert network. https://emergency.cdc.gov/han/2020/han00432.asp
Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P et al (2020) Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324(3):259–269
Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F et al (2020) Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 369:m2094
Davies P, Evans C, Kanthimathinathan HK, Lillie J, Brierley J, Waters G et al (2020) Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health 4(9):669–677
Lee PY, Day-Lewis M, Henderson LA, Friedman KG, Lo J, Roberts JE et al (2020) Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Investig. https://doi.org/10.1172/JCI141113 (Epub ahead of print. PMID: 32701511)
Pilania RK, Bhattarai D, Singh S (2018) Controversies in diagnosis and management of Kawasaki disease. World J Clin Pediatr 7(1):27–35
Dionne A, Dahdah N (2018) Myocarditis and Kawasaki disease. Int J Rheum Dis 21(1):45–49
Pilania RK, Jindal AK, Bhattarai D, Naganur SH, Singh S (2020) Cardiovascular involvement in Kawasaki disease is much more than mere coronary arteritis. Front Pediatr 8:526969. https://doi.org/10.3389/fped.2020.526969
Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, Milner JD (2020) Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 324(3):294–296
Ramcharan T, Nolan O, Lai CY, Prabhu N, Krishnamurthy R, Richter AG et al (2020) Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol 41(7):1391–1401. https://doi.org/10.1007/s00246-020-02391-2
Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S (2020) Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Child Basel Switz 7(7):69. https://doi.org/10.3390/children7070069
Bhat CS, Gupta L, Balasubramanian S, Singh S, Ramanan AV (2020) Hyperinflammatory syndrome in children associated with COVID-19: need for awareness. Indian Pediatr 57(10):929–935
Perez-Toledo M, Faustini SE, Jossi SE, Shields AM, Kanthimathinathan HK, Allen JD et al (2020) Serology confirms SARS-CoV-2 infection in PCR-negative children presenting with Paediatric Inflammatory Multi-System Syndrome. MedRxiv Prepr Serv Health Sci. https://doi.org/10.1101/2020.06.05.20123117
Rostad CA, Chahroudi A, Mantus G, Lapp SA, Teherani M, Macoy L, et al. Serology in children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19. medRxiv. 2020;2020.07.10.20150755.
Reddy M, Singh S, Rawat A, Sharma A, Suri D, Rohit MK (2016) Pro-brain natriuretic peptide (ProBNP) levels in North Indian children with Kawasaki disease. Rheumatol Int 36(4):551–559
McCrindle BW, Cifra B (2018) The role of echocardiography in Kawasaki disease. Int J Rheum Dis 21(1):50–55
Dusad S, Singhal M, Pilania RK, Suri D, Singh S (2020) CT Coronary Angiography studies after a mean follow-up of 3.8 years in children with Kawasaki disease and spontaneous defervescence. Front Pediatr 8:274. https://doi.org/10.3389/fped.2020.00274
Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C et al (2020) Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. https://doi.org/10.1148/radiol.2020202288 (Epub ahead of print)
Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H et al (2020) American College of Rheumatology Clinical Guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. https://doi.org/10.1002/art.41454 (Epub ahead of print)
Pilania RK, Jindal AK, Guleria S, Singh S (2019) An update on treatment of Kawasaki disease. Curr Treat Opt Rheumatol 5(1):36–55
Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Reed J, Posada R et al (2020) Multisystem inflammatory syndrome in children related to COVID-19: A New York City experience. J Med Virol. https://doi.org/10.1002/jmv.26224 (Epub ahead of print)
Ruscitti P, Berardicurti O, Di Benedetto P, Cipriani P, Iagnocco A, Shoenfeld Y, Giacomelli R (2020) Severe COVID-19, another piece in the puzzle of the hyperferritinemic syndrome. An immunomodulatory perspective to alleviate the storm. Front Immunol. 11:1130
Dhanalakshmi K, Venkataraman A, Balasubramanian S, Madhusudan M, Amperayani S, Putilibai S, et al. Epidemiological and Clinical Profile of Pediatric inflammatory multisystem syndrome—temporally associated with SARS-CoV-2 (PIMS-TS) in Indian children. Indian Pediatr. 2020 (Epub ahead of print)
Bell DM, Brink EW, Nitzkin JL, Hall CB, Wulff H, Berkowitz ID et al (1981) Kawasaki syndrome: description of two outbreaks in the United States. N Engl J Med 304(26):1568–1575
McIntosh K (2005) Coronaviruses in the limelight. J Infect Dis 191(4):489–491
Jordan-Villegas A, Chang ML, Ramilo O, Mejías A (2010) Concomitant respiratory viral infections in children with Kawasaki disease. Pediatr Infect Dis J 29(8):770–772
Huang X, Huang P, Zhang L, Xie X, Xia S, Gong F et al (2015) Influenza infection and Kawasaki disease. Rev Soc Bras Med Trop 48(3):243–248
Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS (2005) Association between a novel human coronavirus and Kawasaki disease. J Infect Dis 191(4):499–502
Shimizu C, Shike H, Baker SC, Garcia F, van der Hoek L, Kuijpers TW et al (2005) Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J Infect Dis 192(10):1767–1771
Dominguez SR, Anderson MS, Glodé MP, Robinson CC, Holmes KV (2006) Blinded case-control study of the relationship between human coronavirus NL63 and Kawasaki syndrome. J Infect Dis 194(12):1697–1701
Lehmann C, Klar R, Lindner J, Lindner P, Wolf H, Gerling S (2009) Kawasaki disease lacks association with human coronavirus NL63 and human bocavirus. Pediatr Infect Dis J 28(6):553–554
Shirato K, Imada Y, Kawase M, Nakagaki K, Matsuyama S, Taguchi F (2014) Possible involvement of infection with human coronavirus 229E, but not NL63 in, Kawasaki disease. J Med Virol 86(12):2146–2153
Rivas MN, Wakita D, Franklin MK, Carvalho TT, Abolhesn A, Gomez AC et al (2019) Intestinal permeability and IgA provoke immune vasculitis linked to cardiovascular inflammation. Immunity 51(3):508-521.e6
Rowley AH, Wylie KM, Kim KYA, Pink AJ, Yang A, Reindel R et al (2015) The transcriptional profile of coronary arteritis in Kawasaki disease. BMC Genom 16(1):1076
Wright VJ, Herberg JA, Kaforou M, Shimizu C, Eleftherohorinou H, Shailes H et al (2018) Diagnosis of Kawasaki disease using a minimal whole-blood gene expression signature. JAMA Pediatr 172(10):e182293–e182293
Popper SJ, Watson VE, Shimizu C, Kanegaye JT, Burns JC, Relman DA (2009) Gene transcript abundance profiles distinguish Kawasaki disease from adenovirus infection. J Infect Dis 200(4):657–666
Hoang LT, Shimizu C, Ling L, Naim ANM, Khor CC, Tremoulet AH et al (2014) Global gene expression profiling identifies new therapeutic targets in acute Kawasaki disease. Genome Med 6(11):541
Giordani L, Quaranta MG, Marchesi A, Straface E, Pietraforte D, Villani A et al (2011) Increased frequency of immunoglobulin (Ig)A-secreting cells following Toll-like receptor (TLR)-9 engagement in patients with Kawasaki disease. Clin Exp Immunol 163(3):346–353
Xu M, Jiang Y, Wang J, Liu J, Liu C, Liu D et al (2019) Distinct variations of antibody secreting cells and memory B cells during the course of Kawasaki disease. BMC Immunol 20(1):16
Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC (2001) Oligoclonal IgA Response In The Vascular Wall In Acute Kawasaki Disease. J Immunol 166(2):1334–1343
Rowley AH, Shulman ST, Garcia FL, Guzman-Cottrill JA, Miura M, Lee HL et al (2005) Cloning the arterial IgA antibody response during acute Kawasaki disease. J Immunol 175(12):8386–8391
Roncati L, Ligabue G, Fabbiani L, Malagoli C, Gallo G, Lusenti B et al (2020) Type 3 hypersensitivity in COVID-19 vasculitis. Clin Immunol 217:108487
Blanchard JN, Powell HC, Freeman WR, Letendre S, Blanchard D, Shimizu C et al (2003) Recurrent Kawasaki disease—like syndrome in a patient with acquired immunodeficiency syndrome. Clin Infect Dis 36(1):105–111
Johnson RM, Bergmann KR, Manaloor JJ, Yu X, Slaven JE, Kharbanda AB (2016) Pediatric Kawasaki disease and adult human immunodeficiency virus Kawasaki-like syndrome are likely the same malady. Open Forum Infect Dis 3(3):ofw160. https://doi.org/10.1093/ofid/ofw160
Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C et al (2020) Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Investig. https://doi.org/10.1172/JCI140970
Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J et al (2020) Multisystem inflammatory syndrome in children in New York State. N Engl J Med 383(4):347–358
Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D et al (2020) Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care 10(1):69
Nasir A, Al Tatari H, Hamdan MA (2012) Very high serum ferritin levels in three newborns with Kawasaki-like illness. Paediatr Child Health 17(4):201–204
Carnevale S, Beretta P, Morbini P (2020) Direct endothelial damage and vasculitis due to SARS-CoV-2 in small bowel submucosa of COVID-19 patient with diarrhea. J Med Virol. https://doi.org/10.1002/jmv.26119 (Epub ahead of print)
Gruber C, Patel R, Trachman R, Lepow L, Amanat F, Krammer F, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). medRxiv. 2020;2020.07.04.20142752.
Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L et al (2020) The Immunology of multisystem inflammatory syndrome in children with COVID-19. Cell S0092–8674(20):31157
Sakurai Y (2019) Autoimmune aspects of Kawasaki DISEASE. J Investig Allergol Clin Immunol 29(4):251–261
Manson JJ, Crooks C, Naja M, Ledlie A, Goulden B, Liddle T et al (2020) COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol 2(10):e594-602
Webb BJ, Peltan ID, Jensen P, Hoda D, Hunter B, Silver A et al (2020) Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. https://doi.org/10.1016/S2665-9913(20)30343-X (Epub ahead of print)
Sokolovsky S, Soni P, Hoffman T, Kahn P, Scheers-Masters J (2020) COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2020.06.053 (Epub ahead of print)
Shaigany S, Gnirke M, Guttmann A, Chong H, Meehan S, Raabe V et al (2020) An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet 396(10246):e8-10
Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S et al (2020) Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep 69(40):1450–1456. https://doi.org/10.15585/mmwr.mm6940e1
Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y et al (2020) Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 26(4):502–505
Cohen R, Jung C, Ouldali N, Sellam A, Batard C, Cahn-Sellem F, et al. Assessment of spread of SARS-CoV-2 by RT-PCR and concomitant serology in children in a region heavily affected by COVID-19 pandemic. medRxiv. 2020;2020.06.12.20129221.
Lassaunière R, Frische A, Harboe ZB, Nielsen AC, Fomsgaard A, Krogfelt KA et al (2020) Evaluation of nine commercial SARS-CoV-2 immunoassays. Medrxiv. https://doi.org/10.1101/2020.04.09.20056325
Long Q-X, Liu B-Z, Deng H-J, Wu G-C, Deng K, Chen Y-K et al (2020) Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26(6):845–848
Gazzaruso C, Carlo Stella N, Mariani G, Nai C, Coppola A, Naldani D et al (2020) High prevalence of antinuclear antibodies and lupus anticoagulant in patients hospitalized for SARS-CoV2 pneumonia. Clin Rheumatol 39(7):2095–2097
Funding
Nil.
Author information
Authors and Affiliations
Contributions
JK and RKP prepared the first draft of manuscript, figures and tables. JK and RKP shared the first authorship. RK and SK provided expert inputs; SS and DD involved in all the stages from structuring, guiding, planning and critically revising the review. All the authors have substantially contributed in drafting the manuscript, and all the authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer
We hereby state that this manuscript is original and no part of the manuscript has either been published before, or is under consideration of publication elsewhere. The definition of scientific term alluded to, in the manuscript have been duly cited.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kabeerdoss, J., Pilania, R.K., Karkhele, R. et al. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int 41, 19–32 (2021). https://doi.org/10.1007/s00296-020-04749-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04749-4