Abstract
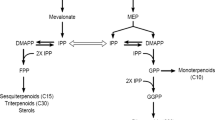
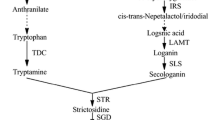
Jasmonates enhance the expression of various genes involved in terpenoid indole alkaloid (TIA) biosynthesis in Catharanthus roseus. We applied precursor feeding to our C. roseus suspensions to determine how methyl jasmonate (MJ) alters the precursor availability for TIA biosynthesis. C. roseus suspensions were induced with MJ (100 μM) on day 6 and fed loganin (0.30 mM), tryptamine (0.15 mM), loganin plus tryptamine, or geraniol (0.1–1.0 mM) on day 7. While MJ increased ajmalicine production by 3-fold, induced cultures were still limited by terpenoid precursors. However, both induced and non-induced cultures became tryptamine-limited with excess loganin. Geraniol feeding also increased ajmalicine production in non-induced cultures. But MJ appeared to increase geraniol availability in induced cultures, due presumably to the increased expression of Dxs with MJ addition.



Similar content being viewed by others
References
Aerts RJ, Gisi D, De Carolis E, De Luca V, Bauman TW (1994) Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloids in Catharanthus and Cinchona seedlings. Plant J 5:635–643
Collu G, Unver N, Peltenburg-Looman AMG, van der Heijden R, Verpoorte R, Memelink J (2001) Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett 508:215–220
Collu G, Alonso Garcia A, van der Heijden R, Verpoorte R (2002) Activity of the cytochrome P450 enzyme geraniol 10-hydroxylase and alkaloid production in plant cell cultures. Plant Sci 162(1):165–172
Contin A, van der Heijden R, ten Hoopen HJG, Verpoorte R (1998) The inoculum size triggers tryptamine or secologanin biosynthesis in a Catharanthus roseus cell culture. Plant Sci 139:205–211
El-Sayed M, Verpoorte R (2002) Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tissue Org Cult 68(3):265–270
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:155–158
Gantet P, Imbault N, Thiersault M, Doireau P (1998) Necessity of a functional octadecanoid pathway for indole alkaloid synthesis by Catharanthus roseus cell suspensions cultured in an auxin-starved medium. Plant Cell Physiol 39:220–225
Gundlach H, Muller MJ, Kutchan TM, Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA 89:2389–2393
Hong S-B, Hughes EH, Shanks JV, San K-Y, Gibson SI (2003) Role of the non-mevalonate pathway in indole alkaloid production by Catharanthus roseus hairy roots. Biotechnol Prog 19(3):1105–1108
Lee-Parsons CWT, Ertürk S, Tengtrakool J (2004) Enhancement of ajmalicine production in Catharanthus roseus cell cultures with methyl jasmonate is dependent on timing and dosage of elicitation. Biotechnol Lett 26(20):1595–1599
Memelink J, Verpoorte R, Kijne, W (2001) ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci 6:212–219
Menke FLH, Parchmann S, Mueller MJ, Kijne JW, Memelink J (1999a) Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in Catharanthus roseus. Plant Physiol 119:1289–129
Menke FLH, Champion A, Kijne JW, Memelink J (1999b) A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2. EMBO J 18:4455–4463
Moreno PRH, van der Heijden R, Verpoorte, R (1993) Effect of terpenoid precursor feeding and elicitation on formation of indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Rep 12(12):702–705
Morgan JA, Shanks JV (2000) Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J Biotechnol 79:137–145
Naudascher F, Doireau P, Guillot A, Viel C, Thiersault M (1989a) Time-course studies on the use of secologanin by Catharanthus roseus cells cultured in vitro. J Plant Physiol 134:608–612
Naudascher F, Doireau P, Guillot A, Thiersault M (1989b) Time-course studies on the use of loganin by Catharanthus roseus cells cultured in vitro. J Plant Physiol 135:366–368
Rijhwani SK, Shanks JV (1998) Effect of elicitor dosage and exposure time on biosynthesis of indole alkaloids by Catharanthus roseus hairy root cultures. Biotechnol Progr 14:442–449
Schiel O, Witte L, Berlin J (1987) Geraniol-10-hydroxylase activity and its relation to monoterpene indole alkaloid accumulation in cell suspension cultures of Catharanthus roseus. Z. Naturforsch C 42(9–10):1075–1081
Van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Van der Fits L, Memelink J (2001) The jasmonate-inducible AP2/ERF-domain transcription factor ORCA3 activates gene expression via interaction with a jasmonate-responsive promoter element. Plant J 25:43–53
Whitmer S, Canel C, Hallard D, Goncalves C, Verpoorte R (1998) Influence of precursor availability on alkaloid accumulation by transgenic cell line of Catharanthus roseus. Plant Physiol 116(2):853–857
Whitmer S, van der Heijden R, Verpoorte, R (2002a) Effect of precursor feeding on alkaloid accumulation by a strictosidine synthase over-expressing transgenic cell line S1 of Catharanthus roseus. Plant Cell Tiss Org Cult 69(1):85–93
Whitmer S, van der Heijden R, Verpoorte R (2002b) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus. J Biotechnol 96(2):193–203
Wong PL, Royce AJ, Lee-Parsons CWT (2004) Improved ajmalicine production and recovery from Catharanthus roseus suspensions with increased product removal rates. Biochem Eng J 21:253–258
Acknowledgements
This work was supported in part by the National Science Foundation (NSF-CAREER, Grant No. BES-0134511). A. Royce was supported in part by Giner, Inc. We would like to thank Dr. Robert Verpoorte (University of Leiden, The Netherlands) for the C. roseus cultures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. K. Kamo
Rights and permissions
About this article
Cite this article
Lee-Parsons, C.W.T., Royce, A.J. Precursor limitations in methyl jasmonate-induced Catharanthus roseus cell cultures. Plant Cell Rep 25, 607–612 (2006). https://doi.org/10.1007/s00299-005-0109-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0109-y