Abstract
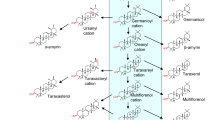
A homology-based PCR method was used to clone a cDNA encoding oxidosqualene cyclase from Centella asiatica, which produces a large quantity of triterpene saponins such as asiaticoside and madecassoside. Sequence analysis of one clone found sequences related to β-amyrin synthase. An open reading frame in the full-length clone was named CabAS (Centella asiatica putative β-amyrin synthase). On the basis of amino acid sequence, CabAS appears to be an enzyme (β-amyrin synthase) that synthesizes β-amyrin. Southern analysis showed that the C. asiatica genome contains one copy of the CabAS gene. Northern blot analysis demonstrated that the CabAS gene is expressed in leaves with no detectable transcript in other plant tissues, consistent with the organ-specific accumulation of the asiaticoside. Up-regulation of expression of CabAS by methyl jasmonate in leaves was also demonstrated.







Similar content being viewed by others
References
Abe I, Rohmer M, Prestwich GD (1993) Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem Rev 93:2189–2206
Abe I, Prestwich GD (1995) Identification of the active site of vertebrate oxidosqualene cyclase. Lipids 30:231–234
Aoyagi H, Kobayashi Y, Yamada K, Yokoyama K, Kusakari K, Tanaka H (2001) Efficient production of saikosaponins in Bupleurum falcatum root fragments combined with signal transducers. Appl Microbiol Biotechnol 57:482–488
Bach TJ, Benveniste P (1997) Cloning of cDNAs or genes encoding enzymes of sterol biosynthesis from plants and other eukaryotes: Heterologous expression and complementation analysis of mutations for functional characterization. Prog Lipid Res 36:197–226
Baisted DJ (1971) Sterol and triterpene synthesis in the developing and germination pea seed. Biochem J 124:375–383
Baek YW (1997) Micropropagation of Centella asiatica (L) Urban by in vitro cultures and production of triterpene glycosides. PhD thesis, Chonnam University, Gwangju
Corey EJ, Cheng H, Baker CH, Matsuda SPT, Li D, Song X (1997) Methology for the preparation of pure recombinant S. cerevisiae lanosterol synthase using a baculovirus expression system. Evidence that oxirane cleavage and A-ring formation are concerted in the biosynthesis of lanosterol from 2,3-oxidosqualene. J Am Chem Soc 119:1277–1288
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Frohman MA, Dush MK, Martin GR (1988) Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA 85:8998–9002
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res 50:195–202
Haralampidis K, Bryan G, Qi X, Papadopoulou K, Bakht S, Melton R, Osbourn A (2001) A new class of oxidosqualene cyclases directs synthesis of antimicrobial phytoprotectants in monocots. Proc Natl Acad Sci USA 98:13431–13436
Hayashi H, Huang PY, Kirakosyan A, Inoue K, Hiraoka N, Ikeshiro Y, Kushiro T, Shibuya M, Ebizuka Y (2001a) Cloning and characterization of a cDNA encoding beta-amyrin synthase involved in glycyrrhizin and soyasaponin biosynthesis in liquorice. Biol Pharm Bull 24:912–916
Hayashi H, Huang P, Inoue K, Hiraoka N, Ikeshiro Y, Yazaki K, Tanaka S, Kushiro T, Shibuya M, Ebizuka Y (2001b) Molecular cloning and characterization of isomultiflorenol synthase, a new triterpene synthase from Luffa cylindrical, involved in biosynthesis of bryonolic acid. Eur J Biochem 268:6311–6317
Hayashi H, Huang PY, Inoue K (2003) Up-regulation of soyasaponin biosynthesis by methyl jasmonate in cultured cells of Glycyrrhiza glabra. Plant Cell Physiol 44:404–411
Hayashi H, Huang P, Takada S, Obinata M, Shibuya M, Ebizuka Y (2004) Differential expression of three oxidosqualene cyclase mRNAs in Glycyrrhiza glabra. Biol Pharm Bull 27:1086–1092
Hostettmann K, Marston A (1995) Saponins. Cambridge University Press, Cambridge
Iturbe-Ormaetxe I, Haralampidis K, Papadopoulou K, Osbourn AE (2003) Molecular cloning and characterization of triterpene synthases from Medicago truncatula and Lotus japonicus. Plant Mol Biol 51:731–743
Kartnig T (1988) Clinical application of Centella asiatica (L.) Urb. In: Craker LE, Simon JE (eds) Recent advances in botany, horticulture and pharmacology, vol 3. Oryx, Phoenix
Kartnig T, Hoffmann-Bohm K (1992) Centella. In: Hänsel R, Keller K, Rimpler H, Schneider G (eds) Hager’s Handbuch der Pharmazeutischen Praxis. Springer, Berlin Heidelberg New York
Kawano N, Ichinose K, Ebizuka Y (2002) Molecular cloning and functional expression of cDNAs encoding oxidosqualene cyclase from Costus speciosus. Biol Pharm Bull 25:477–482
Kim OT, Kim MY, Hong MH, Ahn JC, Oh MH, Hwang B (2002) Production of triterpene glycosides from whole plant cultures of Centella asiatica (L.) Urban. Korean J Plant Biotechnol 29:275–279
Kim OT, Kim MY, Hong MH, Ahn JC, Hwang B (2004) Stimulation of asiaticoside production from Centella asiatica whole plant cultures by elicitors. Plant Cell Rep 23:339–344
Kushiro T, Shibuya M, Ebizuka Y (1998) Beta-amyrin synthase—cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur J Biochem 256:238–244
Kushiro T, Shibuaya M, Masuda K, Ebizuka Y (2000) Mutational studied on triterpene synthases: engineering lupeol synthase into beta-amyrin synthase. J Am Chem Soc 122:6816–6824
Lu MB, Wong HL, Teng WL (2001) Effects of elicitation on the production of saponin in cell culture of Panax ginseng. Plant Cell Rep 20:647–677
Matsuda H, Morikawa T, Ueda H, Yoshikawa M (2001) Medicinal foodstuffs. XXVII. Saponin constituents Gotu Kola (2): Structures of new ursane- and oleanane-type triterpene oligoglycosides, centellasaponin B, C, and D, from Centella asiatica cultivated in Sri Lanka. Chem Pharm Bull 49:1368–1371
Morita M, Shibuya M, Kushiro T, Masuda K, Ebizuka (2000) Molecular cloning and functional expression of triterpene synthases from pea (Pisum sativum)—new alpha-amyrin-producing enzyme is a multifunctional triterpene synthase. Eur J Biochem 267:3453–3460
Nes WR, Heftmann E (1981) A comparison of triterpenoids with sterols as membrane components. J Nat Prod 44:377–400
Pointel JP, Boccalon H, Cloarec M, Ledebehat C, Joubert M (1987) Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology 38:46–50
Poralla K, Hewelt A, Prestwich GD, Abe I, Reipen I, Sprenger G (1994) A specific amino acid repeat in squalene and oxidosqualene cyclases. Trends Biochem Sci 19:157–158
Reinbothe S, Mollenhauer B, Reinbothe C (1994) JIPs and RIPs: the regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell 6:1197–1209
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning—a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
Sembdner G, Parthier B (1993) The biochemistry and the physiological and molecular actions of jasmonates. Annu Rev Plant Physiol 44:569–589
Shibuya M, Zhang H, Endo A, Shishikura K, Kushiro T, Ebizuka Y (1999) Two branches of the lupeol synthase gene in the molecular evolution of plant oxidosqualene cyclases. Eur J Biochem 266:302–307
Staswick PE (1992) Jasmonate, genes, and fragrant signals. Plant Physiol 99:804–807
Suzuki H, Achnine L, Xu R, Matsuda SPT, Dixon RA (2002) A genomic approach to the early stages of triterpene saponin biosynthesis in Medicago truncatula. Plant J 32:1033–1048
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Trojanowska MR, Osbourn AE, Daniels MJ, Threlfall DR (2000) Biosynthesis of avenacins and phytosterols in roots of Avena sativa cv. Image. Phytochemistry 54:153–164
Trojanowska MR, Osbourn AE, Daniels MJ, Threlfall DR (2001) Investigation of avenacin-deficient mutants of Avena strigosa. Phytochemistry 56:121–129
Acknowledgement
This work was supported by a grant from the Biogreen 21 Program, Rural Development Administration, Republic of Korea
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.S. Chung
Rights and permissions
About this article
Cite this article
Kim, O.T., Kim, M.Y., Huh, S.M. et al. Cloning of a cDNA probably encoding oxidosqualene cyclase associated with asiaticoside biosynthesis from Centella asiatica (L.) Urban. Plant Cell Rep 24, 304–311 (2005). https://doi.org/10.1007/s00299-005-0927-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0927-y