Abstract
From 2014 to 2018, we performed three on-site eradication actions of Poa annua occurring on King George Island. We aimed at (1) assessing the population response to eradication efforts, (2) evaluating the campaign success, and (3) identifying the most important factors likely to influence eradication success. The first partial eradication action reduced the initial population of around 1500 tussocks to around 1100 tussocks with less than 4 m2 canopy area. In treated locations, we observed high re-establishment where no soil removal was performed, while only a marginal recruitment where plants were removed with associated soil. In the 2017/2018 season, we recorded over 1800 tussocks, which all were subsequently removed. Performing eradication according to the prescribed scheme (plant and soil removal) should result in eradication success. We evaluate that the probability of successful eradication of the population is high because of small size and number of separate infestation sites, complete spatial and ecological isolation of infestation, high accessibility of target population, and well-known current location of infestation sites. The factors which reduce the likelihood of eradication success are long reaction time, high adaptation of the species to new environmental conditions, and high propagule longevity. Reinvasion possibility and frequent personnel changes in the eradication team resulting in varying levels of personnel awareness and experience may also negatively influence eradication success. An invasion, not managed for many years, may still be targeted, but its successful eradication depends on the “human factor”, which may drive the success of the action in opposing directions.
Similar content being viewed by others
Introduction
The Antarctic remains the only major area on Earth that has been little affected by human activity. The major reason for preserving this pristine area was its harsh environment, which for ages discouraged people from venturing into the inhospitable waters surrounding this continent and postponed the discovery of Antarctica until the nineteenth century. Even then there were no permanent settlements. Intensive human exploitation (hunting for whales and fur seals) threatened, however, the fragile ecosystem (for summary of the history of human influence at Thomas Point Oasis, King George Island—see Galera et al. 2018, Appendix A). Scientific knowledge on preserving natural biotic resources led the international community to preserve the area under the Antarctic Treaty [adopted in 1959, entered into force in 1961, The Antarctic Treaty (2021)]. Based on this agreement, the international community responsible for managing the Antarctic non-ecumene (the uninhabited region, Mayhew 2015) is taking appropriate measures to minimize anthropogenic influences in this region. The provisions successfully regulated the use of natural resources to minimize human impact in this region. The Protocol on Environmental Protection to the Antarctic Treaty [(2021), adopted in 1991, entered into force in 1998] was of particular importance in this matter.
However, in recent decades, the major threat to Antarctic biodiversity emerged from the increasing number of alien species, becoming a particularly significant problem (Chown et al. 2012; Huiskes et al. 2014; Hughes and Convey 2014; Hughes and Pertierra 2016). Intense legislative work has resulted in documents providing the rules of conduct for scientists and regulating the organization of tourist traffic in this region (e.g., Guidelines on Contingency Planning, Insurance and Other Matters for Tourist and Other Non-Governmental Activities in the Antarctic Treaty Area 2017; Information Exchange Requirements 2019; Site Guidelines for Visitors Checklist 2019). According to these provisions, each Antarctic Treaty party is obliged to prevent the conscious and unconscious introduction of alien organisms, and if they are found in the Antarctic—to eradicate them as soon as possible and then monitor the efficacy of eradication programs in follow-up surveys (Non-Native Species Manual 2019; Hughes and Pertierra 2016).
In Antarctica, the number of invasions is still small in comparison with other parts of the globe. The major factors responsible for this insulation are strong spatial and ecological isolation together with harsh environmental conditions (Galera et al. 2018). Harsh climate, strong spatial diversity of edaphic conditions, and limited possibility of local migration between small and spatially isolated terrestrial ecosystems further limit the spread of invasions.
The only known case hitherto of a successful invasion of an alien plant species in the Antarctic is a population of annual bluegrass (Poa annua L.) occurring in Point Thomas Oasis on King George Island (South Shetlands, Maritime Antarctic). Point Thomas Oasis is an area free of ice for one to three months during the year (Antarctic oasis). The size of this oasis is approximately 520 ha. In comparison with spectacular invasions of other species in more hospitable parts of the world, this specific invasion may seem not especially dangerous in terms of ecological and economic consequences. The invasion started relatively recently (first sighting in 1985), has been monitored from the start, and the infested area is still small. For detailed information on the history of invasion, see Galera et al. (2019). Despite this, with the consent of the former Head of the Station Operator in 2014, we initiated the eradication of this population (Galera et al. 2017) fulfilling the responsibilities of the Operator of Arctowski Station imposed by the Antarctic Treaty. This eradication was preceded by detailed study of the species’ ecology in order to better ‘refine the method’ (Wódkiewicz et al. 2013, 2014; Galera et al. 2015). The resulting eradication scheme was described after the initial stage of the eradication (Galera et al. 2017). We subsequently reported on the removal of this species after two years of eradication efforts and the role of seedbank in the survival of the local population (Galera et al. 2019). As the assessment of the possibility of successful eradication is important for the management of other eradication campaigns, we present this paper, which summarizes the response of this population to extirpation efforts. Most importantly, we present our evaluation of the action success and discuss eradication-related factors which influence the outcome of eradication action. Our main goals were, therefore, the following:
-
1.
assessment of the population response to the performed eradication;
-
2.
evaluation of the possibility of campaign success; and
-
3.
an attempt to identify the most important factors influencing the eradication success for the benefit of further actions.
Material and methods
Response of the target population to extirpation
We performed the initial on-site partial eradication actions in 2014/2015 and 2015/2016 Antarctic summer seasons. They were aimed at method development and compared: 1. removal of plants only with 2. removal of plants with associated soil up to 10 cm depth (Galera et al. 2017). After evaluating the scheme to be used, we attempted to eradicate the whole population in the 2017/2018 Antarctic summer season. In order to reduce the seed bank, we have removed the soil from under all eradicated P. annua plants. Eradication actions in the following years were taken over by the personnel of the Station Operator, Institute of Biochemistry and Biophysics PAS.
In the 2014/2015, 2015/2016, and 2017/2018 austral summers, we performed annual bluegrass population censuses in the vicinity of Arctowski Station using a 10 × 10 m grid , as well as in an earlier reported site on the forefield of Ecology Glacier (Galera et al. 2017, 2019). During each of the three performed censuses, the population number was mapped on prepared cartograms. Annual bluegrass tussock was used as the demographic unit for the cartograms. We also noted if the tussocks developed generative (flowering and/or fruiting) shoots (Table 1) and noted the tussock diameter ascribing tussocks to one of five tussock size classes: (0, 1], (1, 5], (5, 10], (10, 15], and more than 15 cm.
We then calculated the canopy area of the tussocks. As we evaluated the tussock size using five size classes, we were able to provide only the minimum and maximum tussock canopy area. This was calculated by multiplying the number of tussocks in each diameter class by individual tussock size based on, respectively, minimum and maximum diameter class limits. For the last size class, we used 20 cm as the maximum tussock diameter. We assumed that tussocks were circular and used their diameter to calculate tussock canopy area. Additionally, we analyzed the percent of individuals starting to flower, i.e., with generative shoots (Table 1). We used two proportion Z test to assess differences in proportion of the smallest individuals, as well as differences in the share of flowering individuals between censuses.
Evaluation of eradication feasibility and assessment of the importance of eradication-related factors
To evaluate eradication feasibility after several years of the action duration, we used a set of 24 factors recognized in the literature as affecting the probability of eradication of an alien plant species (for definitions of factors and main literature sources see Online Resource 1 in Electronic Supplementary Material). A simple ordinal scale (0 to 3) expresses subjective factor impact on eradication success, where 0 indicates no impact on eradication success and 3 indicates high impact (Table 2). A factor promoting eradication success is additionally assigned a “+” symbol and a factor restricting eradication is assigned a “−” symbol. An “X” symbol indicates that it is difficult to evaluate the influence of a factor or the factor may drive eradication success in contrasting directions.
Results
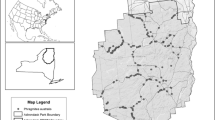
The annual bluegrass population at Point Thomas Oasis forms two discrete subpopulations. A much larger subpopulation (1813 tussocks in 2017/2018 Antarctic season) is found in the vicinity of the Polish Antarctic Station (Fig. 1), where the species has been recorded for over 35 years. The second subpopulation appeared about 1.5 km away on the moraines of the Ecology Glacier much later in the 2008/2009 Antarctic season and in 2017/2018 had 54 tussocks.
Response of target population size to extirpation action
The initial size of the target population in 2014/2015 before the eradication action commenced was around 1500 tussocks, which had an estimated canopy area of 1.13 to 4.29 m2 (Table 1). During the 2014/2015 austral summer, we extirpated 24% of the population, 314 tussocks in the vicinity of the Station, and all detected 49 tussocks on the Ecology Glacier forefield (Galera et al. 2017) diminishing the size of the target population to around 1100 tussocks of total canopy area of 1.03 to 3.86 m2. Only partial treatment was implemented to assess whether the eradication scheme may promote population expansion. During the next austral summer, the population size was higher (Table 1); however, both the tussock number and canopy size increased in untreated quadrats (i.e., quadrats from which P. annua was not been removed during the 2014/2015 season). In the treated grid squares, we observed a relatively high re-establishment (220 individuals appeared in 2015/2016 season) under the “removal of plants only” eradication scheme, while only a marginal recruitment of individuals (only one seedling—data from the vicinity of the Station) in squares treated according to “removal of plants together with associated soil” eradication scheme (Galera et al. 2019). This indicated that the developed eradication scheme should prove effective. During the second experimental eradication in 2015/2016 austral summer, we extirpated 22% of the population, i.e., 296 tussocks in the vicinity of the Station and 6 tussocks on the Ecology Glacier forefield (Galera et al. 2019). The size of the target population after this treatment diminished to 1473 tussocks with an estimated canopy area between 1.88 and 6.75 m2.
In the 2017/2018 season, we recorded over 1800 tussocks with an estimated canopy of 1.56 to 5.25 m2 (Table 1). We also found tussocks on the Ecology Glacier forefield as well as in 25 grid squares in the vicinity of Arctowski station, in which the species had not been noted for at least 25 years (Fig. 1, squares: D23, D24, F14, F17, G13, G14, G16, G17, H14, H16, H17, I8, I9, I15, I16, J3, J9, J15, K11, L13, M6, N11, N13, O13, U8). All the found tussocks were removed according to the “removal of plants together with associated soil” extirpation scheme (Galera et al. 2017, 2019).
Changes of tussock size and flowering between censuses
The initial 2014/2015 census revealed that the smallest tussocks (up to 1 cm diameter) constituted 36% of the population. Initially the largest tussocks (diameter of more than 15 cm) constituted 1.4% of the population. We did not detect tussocks with a diameter larger than 20 cm. During the course of extirpation, the fraction of smallest tussocks decreased to 20% in 2015/2016, but in 2017/2018 it increased to 46%. The proportion of smallest tussocks was significantly different in both years from the initial census of 2014/2015 (Z test p < 0.0001) (Fig. 2).
The percent of tussocks with generative shoots was 44% in 2014/2015 (Table 1). In 2015/2016, the proportion of flowering individuals was significantly higher (p < 0.0001), but in 2017/2018 returned to the proportion observed during the first census (Table 1). In each of the census years a small percent of the flowering individuals (3.8%, 4.4% and 0.2%, respectively, for each census year) constituted individuals forming the smallest tussocks.
Factors influencing eradication feasibility
Size, location, and monitoring of target population
Prior to the beginning of any eradication action, the size of the P. annua population at Point Thomas Oasis was less than 1500 tussocks with a canopy area under 5 m2 (minimum infestation size) which were scattered over an area of 0.76 ha (maximum infestation size—Table 2, factor 1). The number of tussocks together with the canopy area of the species decreased where partial eradication according to the “removal of plants with associated soil” was performed in consecutive years. The target population was divided into two subpopulations. This is a small number and should not negatively influence the success of eradication action (Table 2, factor 2).
Point Thomas Oasis is fully isolated from other sites permitting plant growth on King George Island by Ecology Glacier and Dara Icefall (Galera et al. 2018; Pudełko et al. 2018). This isolation of infestation (Table 2, factor 3) restricts the spread of the species beyond the Oasis and positively influences eradication success. At the same time, isolation restricts the maximum possible monitoring area (Table 2, factor 4) to the size of the Oasis, which was 524 ha in 2018. Due to possible dispersal of the species, this area should be monitored once or twice during the vegetative season (Table 2, factor 5). At present the monitoring area should cover the 4.8 ha in the vicinity of Arctowski Station (Fig. 1) and the Ecology Glacier forefield site, which should be monitored biweekly (Table 2, factor 5). The recommendation for such frequent monitoring is due to the fact that P. annua can bloom under the snow (Galera et al. 2019). As the snow melts, tussocks that are already in the flowering stage are gradually exposed. It can be expected that the fruits can mature within 2 weeks. However, considering the short Antarctic summer the monitoring intensity should not present any difficulties.
Ease of access to the infested area
Land use and the land management mosaic in the case of P. annua invasion in Point Thomas Oasis should not restrict the eradication action (Table 2, factor 6). On the other hand, accessibility to the site (in terms of the physical distance between the infestation and the place where the eradication action is organized) can be considered in two ways. The main management office is located in Warsaw—14,000 km from the infestation. The second office is the field base—Polish Antarctic Station, in the vicinity of which the infestation is located. We assumed that when conducting field work, the distance to the nearest office is more important. The distance from the Station buildings to the target population is negligible and eradication actions do not require any additional travel (Table 2, factor 7).
Fitness and fecundity of the invasive population
Annual bluegrass is a cosmopolitan species. Due to huge phenotypic plasticity, it is able to thrive under Antarctic conditions (Galera et al. 2015). As well as other invasive plants in harsh environments, it is capable of shifting from annual to biennial growth habit (Williams et al. 2019a; March-Salas and Pertierra 2020). The ability of the species to produce seed, disperse, and recruit in Point Thomas Oasis results in a relatively high climate match for the Antarctic P. annua population (Table 2, factor 8). Due to the lack of large terrestrial herbivores, annual bluegrass has no natural enemies in Antarctica. It is also not exposed to competition from native plants (Galera et al. 2018, Table 2, factor 8b).
The pre-reproductive period of P. annua in the Antarctic seems to be very short, as we noted small individuals (diameter less than 1 cm) are beginning to flower. While several seed production events during one vegetative season (Tutin 1957) are unlikely under Antarctic conditions, small flowering individuals may be overlooked during monitoring and subsequently disperse seed. Additionally, during the 30-year presence of the species at the site, a sufficiently large soil seed bank is likely to have built up to hinder the eradication. Species traits linked to seed production, dispersal, and persistence may, therefore, negatively impact eradication success (Table 2, factors 9–12), although the possibility of vegetative reproduction is minimal (Bond et al. 2007; Galera et al. 2019).
Detectability
Despite the small number of vascular plant species and scarce vegetation at Point Thomas Oasis, factors associated with the target species’ detectability reduce the probability of successful eradication (Table 2, factors 13 and 14). The seasonal timing of removal should enable distinguishing of the target species from other species and should be performed prior to seed set (e.g., Coulston 2002; Panetta and Timmins 2004; Dodd et al. 2015). This may present a problem as in Antarctica some P. annua individuals begin to flower prior to snow melt and upon snow cover melt down plants with fully developed inflorescences may be observed (Galera et al. 2019). This indicates that the annual period during which the species is detectable prior to seed set may be very short and in a negative way affect the probability of eradication success. Additionally, small annual bluegrass individuals may be confused with small individuals of the native Antarctic hairgrass (Deschampsia antarctica Desv.). Both species coincide in space and time and exhibit high variability in shoot color.
Knowledge of the invasive population
Prior to the main eradication action in 2017/2018 austral summer and during the first two seasons the eradication scheme was being prepared, we studied the target population in order to prepare for an informed eradication scheme. Our findings are published in accessible scientific journals and information regarding the extirpation scheme, location (GPS coordinates), and abundance of the local population at the beginning of the action and locations from where the species has been removed is available (Galera et al. 2017, 2019; Table 2, factor 15). We also showed that the biology of the invasive population of P. annua on Thomas Point Oasis is distinct from other populations of this species (Wódkiewicz et al. 2014, 2018; Galera et al. 2015, 2021; Rudak et al. 2018, 2019; Table 2, factor 16).
Knowledge about the feasibility of eradicating the species from other areas is limited (Table 2, factor 17). Successful eradications in the Antarctic pertained to small numbers of individuals (Chwedorzewska et al. 2015; Molina-Montenegro et al. 2015). Research on the control of invasive population on sub-Antarctic Macquarie Island is still ongoing (Williams et al. 2016, 2018, 2019b). Eradication attempts in the Southern Ocean Snares Island have failed (Shaw 2013).
Staff cognition and eradication resources
In the case of P. annua control attempts in Point Thomas Oasis, the long reaction time is a problem (Table 2, factor 18). The invader was first recorded in the 1985/1986 Antarctic summer season. The dynamics of the species’ distribution was monitored (see distribution maps in Galera et al. 2019), but preparations for eradication began only in 2014/2015 (Galera et al. 2017). In the meantime, a large seed bank has developed, which is a major obstacle to the eradication efforts (Table 2, factor 9).
We considered manual removal of plants together with removal of seed-infected soil as the most appropriate method of eradication (Galera et al. 2017, 2019; Table 2, factor 19). The use of biological (Hughes and Pertierra 2016) and chemical (Williams et al. 2019b) methods is questionable due to legal restrictions and unknown impact on the local environment.
All work associated with the devised eradication scheme is simple and may be carried out by staff working at the Station. However, this does not mean that work related to the eradication is performed by the same personnel. The composition of Arctowski's team changes significantly from year to year, which creates a danger that the eradication will be carried out by people unprepared to carry out such tasks. Therefore, the impact of personnel awareness (Table 2, factor 20) may be variable and difficult to estimate. Cognition and sense of responsibility of seasonal workers may be lower than that of ecologists better acquainted with the mechanisms of biological invasions. Personal attitude of people conducting the action may impact the influence of this factor on the success of eradication in different directions.
The transport of personnel and materials needed to perform the action should not constitute an additional heavy burden for the Station Operator as they are small in comparison with the overall costs associated with the operation of the Station (factor 22, Table 2). Additionally, annual bluegrass has no economic value hindering the decision on its eradication (Table 2, factor 23). We can, therefore, summarize that economic problems should not hinder the eradication.
Reinvasion possibility
Fruits and remains of spikelets of P. annua found on clothes and equipment of the Antarctic Expedition participants (Lityńska-Zając et al. 2012) are the direct evidence of the existence of propagule pressure of this species. The local Point Thomas Oasis population may have experienced intraspecific admixture in the past indicating repeated introductions (Wódkiewcz et al. 2018). Therefore, further propagule pressure cannot be excluded nor neglected. Although procedures limiting the import of propagules from external sources into Antarctica are available (Non-Native Species Manual 2019), annual bluegrass is potentially able to penetrate this barrier as the caryopses are small. Therefore, we evaluated the reinvasion possibility (Table 2, factor 24) as medium.
Discussion
Reaction of the invasive population to extirpation
During the first two years of eradication method development, we removed around 20% of tussocks yearly. Partial eradication was performed in order to assess different eradication schemes on recruitment in the population. In most cases, our eradication scheme involving removal of soil from under the extirpated tussocks in order to remove the soil seed bank proved to be successful as in the following year tussocks reappeared mostly in locations where soil had not been removed (Galera et al. 2019). The increase in population size after the first eradication action performed in 2014/2015 was very likely because soil disturbance during the removal of plants, unassisted by soil removal, often results in an increased recruitment of seedlings. Literature data indicate that germination of P. annua is promoted by light (Warwick 1979; Hutchinson and Seymour 1982) therefore seed excavation may promote their germination. The population increase observed in 2015/2016 austral summer season was detected in squares where soil had not been removed during extirpation and in locations where the action had not been performed. This indicates that soil removal is crucial in eradication efforts and soil disturbance enhances recruitment from the soil seed bank. However, the high percent of flowering individuals accompanying the population increase may indicate favorable overall weather conditions during the 2015/2016 season.
Our data from the 2017/2018 growing season indicated that there was a shift in population size structure. We recorded an increase in the percentage of small individuals. This could be a result of successive removal of larger plants coupled with recruitment of individuals from the soil seed bank. We however also recorded large tussocks in 2017/2018. These could result either from missing tussocks during earlier eradication actions due to difficulties in working in the field under Antarctic conditions or enhanced recruitment in locations where soil had not been completely removed. As we did not participate in the eradication action performed during 2016/2017 (actions performed by IBB PAS personnel) we are unable to fully evaluate the causes leading to the population structure observed during the 2017/2018 census. For the action to be successful and declared so a detailed and careful extirpation of plants coupled with sufficient monitoring has to be performed for the coming years.
Factors affecting the success of eradication of Poa annua from Point Thomas Oasis
Size, location, and monitoring of target population
The area occupied by annual bluegrass at Point Thomas Oasis is relatively small, and according to Rejmánek and Pitcairn (2002), should not present a problem for the eradication of this species. This area is also smaller (or comparable) than in the case of another difficult to extirpate population of chickweed (Stellaria media (L.)Vill.) on Macquarie Island (Williams et al. 2019a). The Macquarie Island Stellaria media population was distributed into eight subpopulations, and still evaluated as possible to eradicate. Annual bluegrass at Point Thomas Oasis is distributed into two subpopulations, therefore its eradication should be feasible.
Infestation isolation was indicated as a factor positively influencing the eradication success of procumbent pearlwort (Sagina procumbens L.) on Gough Island (Cooper et al. 2011; Visser et al. 2010) and Stellaria media on Macquarie Island (Williams et al. 2019a). For both species however eradication success was not yet archived. Annual bluegrass at Point Thomas Oasis is even more isolated than both abovementioned invasions. The infestation is not covering the whole King George Island, but only the Oasis due to snow cap cover.
Ease of access to the infested area
Lack of land ownership [Antarctic Treaty has frozen the claim to territorial sovereignty, see Article IV of The Antarctic Treaty (2021)] and residing human population has also been considered as promoting eradication (Shaw 2013). Eradication is also promoted by specific legal regulations of the Antarctic Treaty (2021). The population thrives on a flat and well accessible terrain in contrast to Stellaria media and Sagina procumbens populations (Williams et al. 2019a; Shaw 2013; Visser et al. 2010; Cooper et al. 2011). Therefore, we evaluated this factor as rather promoting than obstructing eradication.
Fitness and fecundity of the invasive population
In comparison with other more hospitable environments the fecundity and population performance of P. annua under Antarctic conditions is relatively low. Total yearly seed production at Point Thomas Oasis was estimated at around 100 thousand seeds with germination capacity based on our earlier findings: 1500 tussocks (Galera et al. 2015) × 3.5 individuals per tussock (Rudak et al. 2019) × 4 generative shoots per individual (Galera et al. 2015) × 4.6 germinable seeds per generative shoot (Rudak et al. 2018). It is much smaller than under temperate climate (150–650 thousand seeds per m2 per year, Lush 1988a). At the same time our estimate of seed production is in accordance with the seed rain in the vicinity of Arctowski Station estimated at 102.6 thousand seeds (76 grid squares of 100 m2 × 13.5 seeds m−2; Galera et al. 2021). Out of 165 caryopses extracted from 20 panicles collected at Point Thomas Oasis only 3% were fully developed and 53% immature, but with possibility to germinate (Rudak et al. 2018). The soil seed bank of the species under optimal conditions can reach up to 210 thousand seeds m−2 (Lush 1988b). While the soil seed bank under Antarctic conditions is spatially highly variable and can reach a median seed density up to around 6000 seeds m−2 underneath tussocks (Wódkiewicz et al. 2014) and 200–400 seeds m−2 in random places away from tussocks (Galera et al. 2021), it is comparable to the soil seed bank of native species (Wódkiewicz et al. 2013). The concentration of seeds underneath the tussocks promotes eradication, as the majority of the soil seed bank can be removed during extirpation and plant presence may indicate the location of a soil seed store.
Under temperate climate conditions seeds may remain viable for up to four years (Warwick 1979). While under sub Antarctic conditions seeds were found to persist for only around two years (Williams et al. 2016), our studies indirectly indicate that in the Antarctic the viability of seeds may be much longer, reaching even up to 16 years (Galera et al. 2021). The significance of the soil seed bank persistence for eradication outcome has also been reported for Stellaria media (Williams et al. 2019a). The presence of the soil seed bank is the main restriction to the studied eradication.
Detectability
Similarly, as in the case of Stellaria media on Macquarie Island (Williams et al. 2019a), detection of annual bluegrass at Point Thomas Oasis may be a pivotal drawback in the removal of the target species, especially when performed by undertrained personnel. This is because of the presence of similar looking species. Therefore we recommend that the extirpation action is performed by adequately trained personnel (preferably botanists). We also advise that the first treatment during each consecutive season to be performed immediately after snow melt as annual bluegrass seeds may ripen under snow cover and disperse shortly after snow melt.
Knowledge of the invasive population
Data on the exact location and biology of local population and the appropriate eradication scheme (e.g., Wódkiewicz et al. 2014; Galera et al. 2017) are available and should be consulted during the forthcoming actions. Although the understanding of the biology of the target population is quite good and may promote eradication, the experience gained during eradications achieved outside Antarctic might not influence much the probability of eradication of the species in Point Thomas Oasis because of different climate-species interactions. To our knowledge, no one has yet started eradicating an invasive population of P. annua larger than a few individuals. Nobody has gained experience in this area, which at least does not facilitate eradication.
Staff cognition and eradication resources
While the removal of a few tussocks in other Antarctic invasions of this species proved to be relatively easy and resulted in successful eradication (Chwedorzewska et al. 2015; Molina-Montenegro et al. 2015), here the long reaction time makes eradication more difficult. On the other hand applicable control methods are well defined and with due care should eventually prove to be effective.
Even short reaction time and the use of various control methods (including non-specific ones) do not guarantee the success of eradication of an invasive species. An instructive example in this regard is the failed eradication of Sagina procumbens from Gough Island. Despite a very short reaction time (first control attempts were made in the year of discovery, Cooper et al. 2011), the small size of the infected area (1.2–1.6 ha in 2010) and the use of diverse control methods (Visser et al. 2010), the species could not be eradicated (Tucker and Underwood 2016; Knapp et al. 2019). One of the causes of the failure to eradicate Sagina procumbens from Gough Island may be frequent changes in the personnel composition of the eradication team, and probably the diverse level of responsibility and experience of people, especially volunteers, involved in the action (Cooper et al. 2011). A similar situation may arise with the P. annua invasion in Point Thomas Oasis. During the 2016/2017 Antarctic growing season and from 2018/2019 onwards, employees of the Institute of Biochemistry and Biophysics PAS continued the eradication action without our participation.
Rejmánek and Pitcairn (2002) indicated that a professionally conducted eradication action should end successfully in the case of a small target population. The field work associated with eradication action is frequently performed by specialists conducting research on the invasion. This was also the case of first steps of annual bluegrass eradication at Point Thomas Oasis (Galera et al. 2017). While to perform actions on a greater spatial scale less experienced personnel are engaged, they should be supervised by experienced scientists who train the less experienced personnel and monitor the eradication actions under way. Therefore, for the eradication to prove successful, the “sufficient enthusiasm of project leaders” (Dodd et al. 2015) needs to be accompanied by a sense of responsibility and awareness of the gravity of the situation of all the personnel.
This “human factor” may be the weakest point in the described eradication campaign. Similarly, coordination between concerned parties may be a weak point of the campaign. We began the eradication and outlined the extirpation method during the first two years of the action (Galera et al. 2017, 2019). Further actions should be well monitored and supervised by a plant invasion ecologist. Lack of supervision for the first 30 years led to the establishment of this population and may be explained by the propensity of animal oriented research programs conducted at Arctowski Station, low interest in botany due to the small number of vascular plants and limited resources for trips to perform the initial screening by ecologists (only grant funding possibilities). Similar problems have been reported by Cooper et al. (2011).
Reinvasion possibility
Even if the current annual bluegrass population at Arctowski is eradicated, new seeds might be imported due to propagule pressure (Lityńska-Zając et al. 2012). “Activities known to increase the risk of biological invasion” in the broader Antarctic (McGeoch et al. 2015) may increase the probability of reinvasion of P. annua. Out of the six invasion drivers, Thomas Point Oasis is exposed to five: tourism, scientific expeditions, the presence of annual residents, the importation of fresh produce and operational airfields. The only exception is the “maintenance of livestock and crops” factor, as there is currently no cultivation in the Oasis.
Despite strict phytosanitary regulations, small seeds may escape detection. Annual bluegrass was reported to have reappeared on Signy Island c.a. 25 years after the species was eradicated (Malfasi et al. 2020). Therefore, reinvasion possibility has to be considered at all times. To control reinvasion, monitoring of the Oasis has to be performed by personnel capable of plant identification.
Assessment of eradication feasibility
Attempts to eradicate invasive species have been more frequently reported in the twenty-first century than earlier, but the question of success of these campaigns is critical. Along with reviewing factors affecting eradication success (e.g.Coulston 2002; Rejmánek and Pitcairn 2002; Howell 2012), the effectiveness of different eradication methods has been studied (Mack and Lonsdale 2002; Flory and Clay 2009; Kettenring and Adams 2011; Simberloff 2014) and causes of failure analyzed (e.g., Gardener et al. 2010; Cooper et al. 2011; Shaw 2013). Experience gained during these actions and their analysis has implications for practice during other eradication actions. Many authors concordantly state that singular eradication action, especially in the presence of a soil seed bank, is ineffective (e.g., Blossey 1999; Tucker and Underwood 2016; Williams et al. 2019b). It has been suggested that invasive plant species eradications need to be performed for at least 10 years (Mack and Lonsdale 2002). Therefore, the importance of long-term planning is critical. However, the exact length of the campaign cannot be predicted accurately.
So far, we have observed an increase in the number of individuals after initial eradication efforts. However this increase has been observed in areas where the eradication has not been performed or was performed in accordance with an inadequate eradication scheme (removal of plants not assisted by soil removal). This likely has led to the recruitment of seedlings from the soil seed bank caused by the disturbance of soil. This result, learnt from partial extirpation during method development phase confirms the efficacy of the developed eradication scheme and informs on possible population increase upon the lack of any actions. Therefore, managers should not give up and further continue the eradication. Further consistent eradication of the seedlings will deplete the seed bank, what should break the increase in population size. One of the pivotal points for successful eradication is that the invasive population does not spread to hard-to-reach areas such as the moss carpet formation and cliffs. An instructive example in this regard is a failed eradication of Sagina procumbens from Gough Island. The most important reasons for this failure are the presence of a seed bank of the species and difficulties in gaining access to some infested sites due to the island’s topography (Visser et al. 2010; Cooper et al. 2011).
Conclusion
The possibility of successful eradication of P. annua from Point Thomas Oasis is high because of small size and number of separate infestation sites, complete isolation of infestation, high accessibility of target population, and well-known current location of infestation sites. The primary factor which reduces the likelihood of success is long reaction time (around 30 years). Also important are relatively high adaptation to new climate conditions and (estimated at 16 years) high seed longevity, medium reinvasion possibility, and rapid personnel changes in the eradication team, which may result in different levels of personnel awareness and preparation in terms of necessary knowledge, skills, and experience needed to manage invasive Antarctic population of P. annua.
We suggest that the eradication of annual bluegrass from Point Thomas Oasis should be divided into two stages: The first is to be several years of extirpation of the standing population (Galera et al. 2017, 2019). The second is at least 10 years of monitoring accompanied by removal of plants recruiting from the soil seed bank. Our findings indicate that while an invasion not managed for many years can still be targeted, the success of such a campaign depends heavily on the “human factor”, which is difficult to evaluate and may drive the success of the action in opposing directions.
Data availability
All new data were presented in the paper.
Code availability
Not applicable.
References
Blossey B (1999) Before, during and after: the need for long-term monitoring in invasive plant species management. Biol Invasions 1:301–311. https://doi.org/10.1023/A:1010084724526
Bond W, Davies G, Turner R (2007) The biology and non-chemical control of annual meadow-grass (Poa annua L.). http://www.gardenorganic.org.uk/weeds/annual-meadow-grass. Accessed 24 May 2017
Chown SL, Lee JE, Hughes KA, Barnes J, Barrett PJ, Bergstrom DM, Convey P, Cowan DA, Crosbie K, Dyer G, Frenot Y, Grant SM, Herr D, Kennicutt MC, Lamers M, Murray A, Possingham HP, Reid K, Riddle MJ, Ryan PG, Sanson L, Shaw JD, Sparrow MD, Summerhayes C, Terauds A, Wall DH (2012) Challenges to the future conservation of the Antarctic. Science 337:158–159. https://doi.org/10.1126/science.1222821
Chwedorzewska KJ, Giełwanowska I, Olech M, Molina-Montenegro MA, Wódkiewicz M, Galera H (2015) Poa annua L. in the maritime Antarctic: an overview. Polar Rec 51:637–643. https://doi.org/10.1017/S0032247414000916
Cooper J, Cuthbert RJ, Gremmen NJM, Ryan PG, Shaw JD (2011) Earth, fire and water: applying novel techniques to eradicate the invasive plant, procumbent pearlwort Sagina procumbens, on Gough Island, a World Heritage Site in the South Atlantic. In: Veitch CR, Clout MN, Towns DR (eds) Island invasives: eradication and management. Occassional Paper SSC 42. IUCN, Gland, pp 162–165
Coulston GJ (2002) Control of invasive plants on the Poor Knights Islands, New Zealand. In: Veitch CR., Clout M (eds) Turning the tide: the eradication of invasive species. IUCN SSC Invasive Species Specialist Group, Gland, pp 164–172
Dodd AJ, Ainsworth N, Burgman MA, McCarthy MA (2015) Plant extirpation at the site scale: implications for eradication programmes. Divers Distrib 21:151–162. https://doi.org/10.1111/ddi.12262
Flory L, Clay K (2009) Invasive plant removal method determines native plant community responses. J Appl Ecol 46:434–442. https://doi.org/10.1111/j.1365-2664.2009.01610.x
Galera H, Chwedorzewska KJ, Wódkiewicz M (2015) Response of Poa annua to extreme conditions: comparison of morphological traits between populations from cold and temperate climate conditions. Polar Biol 38:1657–1666. https://doi.org/10.1007/s00300-015-1731-y
Galera H, Wódkiewicz M, Czyż E, Łapiński S, Kowalska ME, Pasik M, Rajner M, Bylina P, Chwedorzewska KJ (2017) First step to eradication of Poa annua L. from Point Thomas Oasis (King George Island, South Shetlands, Antarctica). Polar Biol 40:939–945. https://doi.org/10.1007/s00300-016-2006-y
Galera H, Chwedorzewska KJ, Korczak-Abshire M, Wódkiewicz M (2018) What affects the probability of biological invasions in Antarctica? Using an expanded conceptual framework to anticipate the risk of alien species expansion. Biodivers Conserv 27:1789–1809. https://doi.org/10.1007/s10531-018-1547-5
Galera H, Rudak A, Czyż EA, Chwedorzewska KJ, Znój A, Wódkiewicz M (2019) The role of the soil seed store in the survival of an invasive population of Poa annua at Point Thomas Oasis, King George Island, maritime Antarctica. Glob Ecol Conserv 19:e00679. https://doi.org/10.1016/j.gecco.2019.e00679
Galera H, Rudak A, Pielech M, Znój A, Chwedorzewska KJ, Wódkiewicz M (2021) Influence of the population spatial structure on seed rain distribution of an invasive plant under harsh environment. Polar Biol 44:587–591. https://doi.org/10.1007/s00300-021-02808-5
Gardener MR, Atkinson R, Renteria JL (2010) Eradications and people: lessons from the plant eradication program in Galapagos. Restor Ecol 18:20–29. https://doi.org/10.1111/j.1526-100X.2009.00614.x
Guidelines on Contingency Planning, Insurance and Other Matters for Tourist and Other Non-Governmental Activities in the Antarctic Treaty Area (2017) Resolution 6 (2017) - ATCM XL - CEP XX, Beijing. Secretariat of the Antarctic Treaty. https://www.ats.aq/devAS/Meetings/Measure/664?s=1&from=1/1/1958&to=1/1/2158&cat=14&top=0&type=0&stat=0&txt=&curr=0&page=1. Accessed 12 Mar 2021
Howell CJ (2012) Progress toward environmental weed eradication in New Zealand. Invasive Plant Sci Manag 5:249–258. https://doi.org/10.1614/IPSM-D-11-00001.1
Hughes KA, Convey P (2014) Alien invasions in Antarctica—is anyone liable? Polar Res 33:22103. https://doi.org/10.3402/polar.v33.22103
Hughes KA, Pertierra LR (2016) Evaluation of non-native species policy development and implementation within the Antarctic Treaty area. Biol Conserv 200:149–159. https://doi.org/10.1016/j.biocon.2016.03.011
Huiskes AHL, Gremmen NJM, Bergstrom DM, Frenot Y, Hughes KA, Imura S, Kiefer K, Marc Lebouvier M, Lee JE, Tsujimoto M, Ware C, Van de Vijver B, Chown SL (2014) Aliens in Antarctica: assessing transfer of plant propagules by human visitors to reduce invasion risk. Biol Conserv 171:278–284. https://doi.org/10.1016/j.biocon.2014.01.038
Hutchinson CS, Seymour GB (1982) Biological flora of the British Isles. Poa Annua L. J Ecol 70:887–901. https://doi.org/10.2307/2260111
Information Exchange Requirements (2019) Decision 7 (2019) - ATCM XLII - CEP XXII, Prague. Secretariat of the Antarctic Treaty. https://www.ats.aq/devAS/Meetings/Measure/700?s=1&from=1/1/1958&to=1/1/2158&cat=14&top=0&type=0&stat=0&txt=&curr=0&page=1. Accessed 12 Mar 2021
Kettenring KM, Adams CR (2011) Lessons learned from invasive plant control experiments: a systematic review and meta-analysis. J Appl Ecol 48:970–979. https://doi.org/10.1111/j.1365-2664.2011.01979.x
Knapp DA, Knapp JJ, Stahlheber KA, Dudley T (2019) A little goes a long way when controlling invasive plants for biodiversity conservation. In: Veitch CR, Clout MN, Martin AR, Russell JC, West CJ (eds) Island invasives: scaling up to meet the challenge. Occasional Paper SSC 62. IUCN, Gland, pp 643–650. https://doi.org/10.2305/IUCN.CH.2019.SSC-OP.62.en
Lityńska-Zając M, Chwedorzewska KJ, Olech M, Korczak-Abshire M, Augustyniuk-Kram A (2012) Diaspores and phyto-remains accidentally transported to the Antarctic Station during three expeditions. Biodivers Conserv 21:3411–3421. https://doi.org/10.1007/s10531-012-0371-6
Lush WM (1988a) Biology of Poa annua in a temperature zone golf putting green (Agrostis stolonifera/Poa annua). I. The above-ground population. J Appl Ecol 25:977–988. https://doi.org/10.2307/2403759
Lush WM (1988b) Biology of Poa annua in a temperate zone golf putting green (Agrostis stolonifera/Poa annua). II. The seed bank. J Appl Ecol 25:989–997. https://doi.org/10.2307/2403760
Mack RN, Lonsdale WM (2002) Eradicating invasive plants: hard won lessons for islands. In: Veitch CR, Clout M (eds) Turning the tide: the eradication of invasive species. IUCN SSC Invasive Species Specialist Group, Gland, pp 164–172
Malfasi F, Convey P, Zaccara S, Cannone N (2020) Establishment and eradication of an alien plant species in Antarctica: Poa annua at Signy Island. Biodivers Conserv 29:173–186. https://doi.org/10.1007/s10531-019-01877-7
March-Salas M, Pertierra LR (2020) Warmer and less variable temperatures favour an accelerated plant phenology of two invasive weeds across sub-Antarctic Macquarie Island. Austral Ecol 45:572–585. https://doi.org/10.1111/aec.12872
Mayhew S (2015) A dictionary of geography, 5th edn. Oxford University Press, Oxford
McGeoch MA, Shaw JD, Terauds A, Lee JE, Chown SL (2015) Monitoring biological invasion across the broader Antarctic: a baseline and indicator framework. Glob Environ Chang 32:108–125. https://doi.org/10.1016/j.gloenvcha.2014.12.012
Molina-Montenegro MA, Pertierra LR, Razeto-Barry P, Díaz J, Finot VL, Torres-Díaz C (2015) A recolonization record of the invasive Poa annua in Paradise Bay, Antarctic Peninsula: modeling of the potential spreading risk. Polar Biol 38:1091–1096. https://doi.org/10.1007/s00300-015-1668-1
Non-Native Species Manual (2019) Revision 2019. Antarctic Treaty Secretariat, Buenos Aires. https://documents.ats.aq/ATCM42/WW/ATCM42_WW008_e.pdf. Accessed 14 Aug 2020
Panetta FD, Timmins SM (2004) Evaluating the feasibility of eradication for terrestrial weed incursions. Plant Prot Q 19:5–11. https://doi.org/10.5194/isprs-archives-XLI-B7-903-2016
Pudełko R, Angiel PJ, Potocki M, Jędrejek A, Kozak M (2018) Fluctuation of glacial retreat rates in the Eastern part of Warszawa Icefield, King George Island, Antarctica, 1979–2018. Remote Sens 10:892. https://doi.org/10.3390/rs10060892
Rejmánek M, Pitcairn MJ (2002) When is eradication of exotic pest plants a realistic goal? In: Veitch CR, Clout M (eds) Turning the tide: the eradication of invasive species. Proceedings of the international conference on eradication of Island Invasives. IUCN SSC Invasive Species Specialist Group, Gland, Cambridge, pp 249–253
Rudak A, Galera H, Znój A, Chwedorzewska KJ, Wódkiewicz M (2018) Seed germination and invasion success of Poa annua L. in Antarctica. Acta Soc Bot Pol 87:3606. https://doi.org/10.5586/asbp.3606
Rudak A, Wódkiewicz M, Znój A, Chwedorzewska KJ, Galera H (2019) Plastic biomass allocation as a trait increasing the invasiveness of annual bluegrass (Poa annua L.) in Antarctica. Polar Biol 42:149–157. https://doi.org/10.1007/s00300-018-2409-z
Shaw JD (2013) Southern Ocean Islands invaded: conserving biodiversity in the world’s last true wilderness. In: Foxcroft LC, Pyšek P, Richardson DM, Genovesi P (eds) Plant invasions in protected areas: patterns, problems and challenges. Invading Nature - Springer series in invasion ecology, vol 7. Springer, Dordrecht, pp 449–470. https://doi.org/10.1007/978-94-007-7750-7_20
Simberloff D (2014) Biological invasions: what’s worth fighting and what can be won? Ecol Eng 65:112–121. https://doi.org/10.1016/j.ecoleng.2013.08.004
Site Guidelines for Visitors Checklist (2019) Resolution 3 (2019) - ATCM XLII - CEP XXII, Prague. Secretariat of the Antarctic Treaty. https://www.ats.aq/devAS/Meetings/Measure/703. Accessed 12 Mar 2021
The Antarctic Treaty (2021) Secretariat of the Antarctic treaty. https://www.ats.aq/e/antarctictreaty.html. Accessed 12 Mar 2021
The protocol on environmental protection to the Antarctic treaty (2021) Secretariat of the Antarctic treaty. https://www.ats.aq/e/protocol.html. Accessed 12 Mar 2021
Tucker GM, Underwood E (2016) Gough Island: an assessment of its status and case for inclusion on the list of world heritage in danger. Report for the Royal Society for the Protection of Birds by the Institute for European Environmental Policy, London
Tutin TG (1957) A contribution to the experimental taxonomy of Poa annua L. Watsonia 4:1–10
Visser P, Louw H, Cuthbert RJ (2010) Strategies to eradicate the invasive plant procumbent pearlwort Sagina procumbens on Gough Island, Tristan da Cunha. Conserv Evidence 7:116–122
Warwick SI (1979) The biology of canadian weeds. 37. Poa annua L. Can J Plant Sci 59:1053–1066
Williams L, Kristiansen P, Sindel BM, Wilson S, Shaw J (2016) Quantifying the seed bank of an invasive grass in the sub-Antarctic: seed density, depth, persistence and viability. Biol Invasions 18:2093–2106. https://doi.org/10.1007/s10530-016-1154-x
Williams LK, Shaw JD, Sindel BM, Wilson SC, Kristiansen P (2018) Longevity, growth and community ecology of invasive Poa annua across environmental gradients in the subantarctic. Basic Appl Ecol 29:20–31. https://doi.org/10.1016/j.baae.2018.02.003
Williams LK, Fergus AJ, Shaw JD, Terauds A, Kristiansen P, Wilson SC, Gosden JL, Ziegler K, Sindel BM (2019a) Quantifying site and species factors to inform the feasibility of eradication of alien plants from Southern Ocean Islands: Stellaria media on Macquarie Island. Biol Invasions 21:993–1005. https://doi.org/10.1007/s10530-018-1880-3
Williams LK, Sindel BM, Kristiansen P, Wilson SC, Shaw JD (2019b) Assessing the efficacy and impact of management of an invasive species in a protected area: Poa annua on sub-Antarctic Macquarie Island. Weed Res 59:180–190. https://doi.org/10.1111/wre.12355
Wódkiewicz M, Galera H, Chwedorzewska KJ, Giełwanowska I, Olech M (2013) Diaspores of the introduced species Poa annua L. in soil samples from King George Island (South Shetlands, Antarctica). Arct Antarct Alp Res 45:415–419. https://doi.org/10.1657/1938-4246-45.3.415
Wódkiewicz M, Ziemiański M, Kwiecień K, Chwedorzewska KJ, Galera H (2014) Spatial structure of the soil seed bank of Poa annua L.—alien species in the Antarctica. Biodiv Conserv 23:1339–1346. https://doi.org/10.1007/s10531-014-0668-8
Wódkiewicz M, Chwedorzewska KJ, Bednarek PT, Znój A, Androsiuk P, Galera H (2018) How much of the invader’s genetic variability can slip between our fingers? A case study of secondary dispersal of Poa annua on King George Island (Antarctica). Ecol Evol 8:592–600. https://doi.org/10.1002/ece3.3675
Acknowledgements
This study was carried out at the Biological and Chemical Research Centre, University of Warsaw, established within a project co-financed by the EU European Regional Development Fund under the Innovative Economy Operational Program, 2007-2013. Part of the material used in the paper was collected at Henryk Arctowski Polish Antarctic Station. We would like to thank Mrs. E.A. Czyż (Remote Sensing Laboratories, Department of Geography, University of Zürich), who updated the distribution map of Poa annua for the Antarctic season 2017/2018. We also thank Dr Ad H.L. Huiskes, Prof. Brian Sindel, and Prof. Dieter Piepenburg (Editor-in-Chief of the Journal) for a detailed and throughout review, language correction suggestions, and valuable comments on our manuscript.
Funding
This study was carried out at the Biological and Chemical Research Centre, University of Warsaw, established within a project co-financed by the EU European Regional Development Fund under the Innovative Economy Operational Program, 2007–2013.
Author information
Authors and Affiliations
Contributions
HG conceived idea, designed research, collected data in the field, and wrote the manuscript. MW analyzed the results and wrote the manuscript. AZ and KJC collected data in the field.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
All authors express their consent to publish the paper.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galera, H., Znój, A., Chwedorzewska, K.J. et al. Evaluation of factors influencing the eradication of annual bluegrass (Poa annua L.) from Point Thomas Oasis, King George Island, Maritime Antarctica. Polar Biol 44, 2255–2268 (2021). https://doi.org/10.1007/s00300-021-02941-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02941-1