Abstract
The left atrium (LA) has a crucial function in maintaining left ventricular filling, which is responsible for about one-third of all cardiac filling. A growing body of evidence shows that LA is involved in several cardiovascular diseases from a clinical and prognostic standpoint. LA enlargement has been recognized as a predictor of the outcomes of many diseases. However, LA enlargement itself does not explain the whole LA’s function during the cardiac cycle. For this reason, the recently proposed assessment of atrial strain at advanced cardiac magnetic resonance (CMR) enables the usual limitations of the sole LA volumetric measurement to be overcome. Moreover, the left atrial strain impairment might allow several cardiovascular diseases to be detected at an earlier stage. While traditional CMR has a central role in assessing LA volume and, through cine sequences, a marginal role in evaluating LA function, feature tracking at advanced CMR (CMR-FT) has been increasingly confirmed as a feasible and reproducible technique for assessing LA function through strain. In comparison to atrial function evaluations via speckle tracking echocardiography, CMR-FT has a higher spatial resolution, larger field of view, and better reproducibility. In this literature review on atrial strain analysis, we describe the strengths, limitations, recent applications, and promising developments of studying atrial function using CMR-FT in clinical practice.
Key Points
• The left atrium has a crucial function in maintaining left ventricular filling; left atrial size has been recognized as a predictor of the outcomes of many diseases.
• Left atrial strain has been confirmed as a marker of atrial functional status and demonstrated to be a sensitive tool in the subclinical phase of a disease.
• A comprehensive evaluation of the three phases of atrial function by CMR-FT demonstrates an impairment before the onset of atrial enlargement, thus helping clinicians in their decision-making and improving patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The function of the left atrium (LA) in modulating left ventricular fillings is very important and can be subdivided into three consecutive phases: (1) a reservoir for pulmonary veins during left ventricle (LV) systole; (2) a conduit for blood flow from pulmonary veins toward LV during LV early diastole; and (3) a booster pump to increase LV fillings during late diastole [1].
Over the past decade, LA size has been widely recognized as an essential marker of adverse cardiovascular events [2]. However, LA enlargement itself does not explain the whole LA function during the cardiac cycle. On the other hand, LA contractile function has been confirmed as a marker of LA functional status and a sensitive tool for detecting early-state diseases [3, 4]
Recently, strain analysis has been proposed as a tool to assess LA phasic function. LA volume and function are traditionally evaluated by echocardiography [4]. In this scenario, speckle tracking echocardiography (STE) was the first modality to assess atrial strain, but it has several limitations, including a suboptimal field of view in the setting of poor acoustic windows and high interobserver variability [5, 6]. Conversely, cardiac magnetic resonance (CMR) is a mainstay in the non-invasive assessment of LA volume and function [7]. Kowallick et al showed the potential of cardiac magnetic resonance feature tracking (CMR-FT) for assessing LA strain and strain rate (SR) parameters [8]. Several studies have demonstrated that LA deformation detected by CMR-FT can allow for an accurate and reproducible analysis of LA function [9, 10].
Another important aspect that needs to be analyzed is the evolving concept of atrial myopathy, first described by Zipes et al [11] as atrial remodeling following atrial fibrillation. Over the past 20 years, the concept of atrial myopathy has evolved with the current consensus definition: “Any complex of structural, architectural, contractile, or electrophysiological changes affecting the atria with the potential to produce clinically relevant manifestations.” [12] According to the EHRAS consensus’s definition, atrial myopathy can also be defined clinically as an abnormality in any of the three aspects of LA function [12].
This review summarizes the current understanding of atrial strain at CMR-FT and provides insights into its clinical application, including its prognostic role.
Atrial strain principles and parameters
LV myocardial strain represents the percentage of longitudinal deformation of a myocardial segment, while strain rate (SR) measures the rate of change in strain during the cardiac cycle [13]. Three different LV myocardial strains are described: longitudinal (i.e., longitudinal shortening from the base to the apex), circumferential (i.e., shortening along the circular perimeter of the myocardium), and radial (the thickening and thinning of the myocardium) [13].
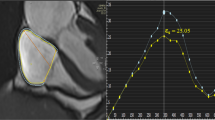
Meanwhile, due to the peculiar fibers’ orientations and the thinness of the atrial wall, only longitudinal strain is usually measured at the atrial level [1]. During the reservoir phase, the LA fills and stretches. It corresponds to a positive atrial strain that reaches its peak before mitral valve opening. Following that, in the conduit phase, there is a negative atrial strain due to passive LA emptying. Finally, in the booster phase, there is a second negative deflection corresponding to atrial systole [1, 14] (Fig. 1, Supplemental Video 1). LA is a dynamic chamber involving a significant interaction between LA function and LV performance during all cardiac cycles. The LA reservoir especially represents atrial relaxation and compliance influenced by the descent of the LV base during systole. LA conduit relies on atrial compliance during ventricular diastole, and it is closely related to LV relaxation and stiffness. Finally, LA booster reflects the intrinsic atrial contractility, which is modulated by the degree of venous return and LV diastolic compliance and pressure [1, 14, 15].
Truong et al investigated normal range values of LA strain and strain rate parameters using CMR-FT in 112 healthy volunteers. The authors reported a mean value of 39.13 ± 9.27, 25.15 ± 8.34, 13.99 ± 4.11, 1.93 ± 0.54, −2.13 ± 0.69, and −2.04 ± 0.61 for reservoir, conduit, and booster strain and strain rate parameters, respectively. In addition, they demonstrated that the reported strain values were comparable between genders, while LA booster and conduit function parameters changed significantly with age [16].
Speckle tracking echocardiography vs. cardiac magnetic resonance
As previously mentioned, the most widely used modality for assessing LA phasic function is echocardiography [15]. STE is an echocardiography technique that tracks natural acoustic markers or “speckles” within the myocardium throughout the cardiac cycle [17]. Many studies have validated the feasibility and reproducibility of STE in studying LA function [18, 19]. STE has several strengths, such as its availability, reasonable cost, and analysis after the ultrasound images have been acquired and stored. Nonetheless, it also has its limitations, including the need for high-quality images, acquisition at a high frame rate, and intra-observer and inter-observer variability. For all these reasons, its clinical application remains somewhat limited [1, 3].
Nowadays, CMR is considered the gold standard imaging modality for evaluating the morphology and function of cardiac chambers, with LA volume and function included [16, 20,21,22]. It offers potential advantages in comparison with echocardiography, namely a wider field of view, high reproducibility, low intra- and inter-observer variability, and high tracking quality [23].
Unfortunately, CMR is limited by its long post-processing time, high cost, limited availability, and incompatibility with claustrophobia or the presence of metal devices inside the body (Table 1). To overcome the long post-processing time, Leng et al investigated the feasibility and effectiveness of a novel semi-automatic method for evaluating long-axis LA strain and strain rate using CMR compared to conventional FT strain analysis [24]. This proposed post-processing method does not require the total segmentation of the LA borders, but it does require the delineation of three distinct anatomical landmarks. The authors reported that semi-automatic LA strain analysis demonstrated excellent correlation and agreement compared to standard FT LA measurements, with a required mean time per subject of 85 ± 10 s (versus 190 ± 12 s using conventional FT analysis) [24].
Atrial strain measurements using CMR-FT remain solely a research tool. One of the limitations for the widespread use of atrial strain analysis may be the limited data on inter-vendor differences. Pathan et al evaluated the inter-vendor comparison of all LA strain parameters using CMR-FT with two types of CMR post-processing software, namely Medis and CVI [25]. The two vendors showed significant differences in mean reservoir (limit of agreement −8.7 to 26.9%), conduit (limit of agreement −9.8%, 21.4%), and booster (limit of agreement −4.7 to 11.3%) in a Bland-Altman analysis. The authors concluded that observed inter-vendor normal reference ranges depend on which vendor is used to compute atrial strain; thus, they suggest cautious use during clinical applications concerning normal reference values [25].
Recently, CMR-FT has been used to quantify atrial deformation with a better accuracy than STE [26, 27]. In this respect, Truong et al demonstrated that, while good tracking was possible in only 91 out of 112 healthy subjects (81%) with STE analysis, tracking using CMR-FT was excellent for the whole cohort, with a good intra- and inter-observer agreement as well [16]. In CMR-FT, steady-state free precession (SSFP) cine CMR images are used and tracked offline to assess myocardial strain.
Clinical application
Atrial strain analysis is progressively developing, owing to steady technological improvements. Thus, data are rapidly emerging regarding the potential role of LA strain analysis in different cardiovascular diseases (see Table 1) characterized by LA enlargement or stretching.
Systemic disease
One of these illnesses is hypertension. In a study by Li et al on hypertensive patients with and without LV hypertrophy [28], LA strain parameters such as LA reservoir and conduit functions were significantly impaired independently of the presence of hypertrophy and in the absence of LA dilatation, thus suggesting that, in high blood pressure cases, strain abnormalities precede structural LA changes [28]. On the contrary, the authors reported that LA booster function was preserved in patients without LV hypertrophy [28].
LA abnormalities were also described in systemic diseases such as diabetes mellitus. In a study by Shao et al investigating atrial strain by CMR-FT in patients with type 2 diabetes [29], LA strains—especially LA global circumferential, LA global radial, and LA global longitudinal function—were reduced in comparison with healthy controls (p < 0.05 for all). The authors reported an improvement in LA strain after diuretic treatments, suggesting a protective effect (or a reversal) in the LA remodeling of these drugs [29]. In addition, LA strain was impaired even in patients with normal LV myocardial strain. These findings suggest that LA impairment may appear earlier than the LV strain abnormalities in the early stage of diabetes mellitus [29].
Cardiomyopathy
Hinojar et al investigated LA contractile function using CMR-FT in seventy-five patients with hypertrophic cardiomyopathy (HCM) in comparison with the same number of healthy controls [3]. The authors also demonstrated impaired LA longitudinal function in the HCM patients with normal LA volume and LV filling pressure in comparison with the healthy control group, with high intra- and inter-observer agreements (r = 0.95 and r = 0.92, respectively) [3]. These findings are consistent with those reported by Yang et al, who found that LA reservoir and conduit impairment occur before LA enlargement in HCM patients [9]. These results imply that LA strain is more sensitive than LA volume in detecting LA involvement in HCM [3, 9].
Moreover, atrial strain using CMR-FT can help in the early detection of cardiac involvement in infiltrative cardiomyopathy due to Anderson-Fabry disease [30]. In the study by Bernardini et al, patients were stratified based on LV involvement. The main finding of the study was that the greater the LV involvement, the more reduced the LA global strain [30]. Additionally, atrial deformation showed a good correlation with native septal T1. The most atrial strain impairment was observed in patients with low T1 values, even in those without any LV hypertrophy or diastolic dysfunction. [30]
LA strain also provides useful information about patients with myocarditis [31, 32]. In a study on 30 patients with clinically proven myocarditis undergoing CMR, Dick et al demonstrated impairments in LA strain parameters during the reservoir and conduit phases [32]. The authors reported that LA early negative peak by SR proved to be the best predictor of acute myocarditis (AUC 0.80), with a sensitivity of 83% and a specificity of 80%, as well as an excellent intra- and inter-observer agreement (ICC = 0.91 and ICC = 0.91, respectively) [32]. Similar results were obtained by Doerner et al, who investigated the usefulness of CMR-FT strain (atrial deformation in particular) as a supplementary tool to the Lake Louise criteria in CMR-based diagnosis of myocarditis [31]. The authors showed that LA early negative peaks at SR and LV global longitudinal strain were very good tools (AUC = 0.72 and AUC = 0.69, respectively) in diagnosing myocarditis. Combining strain parameters and Lake Louise criteria increased the diagnostic accuracy (AUC = 0.77) [31].
Ischemic heart disease
Again, LA contractile function may be altered by ischemic heart disease. In this scenario, Kim et al evaluated the relationship between atrial strain and diastolic dysfunction in patients with myocardial infarction [33]. Their study demonstrated that atrial strain varied significantly according to the grade of LV diastolic dysfunction, with higher sensitivity than LA size [33]. Figure 2 and Supplemental Video 2 show an example of atrial strain in a patient with a history of previous myocardial infarction.
Lapinskas et al evaluated LA strain parameters using CMR-FT in patients with STEMI and secondary mitral regurgitation, demonstrating that all LA strain values—in particular, reservoir, conduit, and booster function strain—and strain rate parameters were significantly impaired in patients with secondary mitral regurgitation; only conduit strain rate parameters significantly increased in patients with mitral regurgitation [34].
Atrial fibrillation
LA dysfunction is the rule in atrial fibrillation. Habibi et al demonstrated that, aside from LA booster function, all LA strain parameters at CMR-FT were significantly more impaired in patients with persistent atrial fibrillation than in healthy subjects, thus supporting the hypothesis of a progressive atrial dysfunction with time [27]. Another study reported that LA strain indices were predictive of the atrial fibrillation onset in patients with risk factors for stroke but without any history of atrial fibrillation, especially patients with LA booster below 17% who had a two-fold increased incidence of AF [35].
Congenital heart disease
Atrial function was also studied in congenital heart disease cases [36, 37]. For example, in thirty-three patients with Fontan circulation, reservoir and conduit strain and strain rate parameters’ functions at CMR-FT were impaired in comparison with age-matched controls. Also, atrial strain was a strong predictor of a worsening in ventricular end-diastolic pressure, cardiac index, exercise capacity, liver stiffness, need for heart transplantation, ventricular assist device, or death [36]. Similar results were shown by Steinmetz et al in 30 patients with Ebstein’s anomaly who shared an impaired quantitative right heart atrio-ventricular deformation at CMR-FT, which is associated with heart failure severity, in terms of the NYHA class [37].
Heart failure with preserved ejection fraction
LA mechanism (above and beyond LA size) was also evaluated in heart failure cases with preserved ejection fraction (HFpEF). von Roeder et al tested the role of LA strain function in HFpEF using CMR, demonstrating that LA reservoir and conduit strain were significantly lower in HFpEF (p = 0.04 and p < 0.01) and that LA conduit strain was the strongest predictor for exercise intolerance on multivariable regression analysis [38]. The authors demonstrated a functional LA remodeling in HFpEF, suggesting that LA conduit strain is an early marker of LA remodeling [38].
Moreover, LA reservoir and conduit strain are also promising prognostic markers in HFpEF. In 101 patients enrolled in a study by Chirinos et al, conduit strain, conduit strain rate, and reservoir strain were associated with increased risks of hospitalization and death [39].
Prognostic role
LA size is linked with the outcomes of many cardiovascular diseases [4]. Guidelines recommend evaluating LA size as a marker of LV filling pressure [40]. LA strain is a promising marker and, likely, a better predictor of outcomes than LA size [4]. In this scenario, several studies have investigated the potential role of atrial strain parameters at CMR in envisaging cardiovascular adverse events [3, 33, 41, 42].
Cardiomyopathy
For example, Hinojar et al evaluated the relationship between LA function and major cardiovascular outcomes—in particular, all-cause death and heart failure—by CMR-FT [3]. The authors found that impairment in longitudinal atrial strain is associated with increased mortality and heart failure onset (p = 0.04 and p = 0.002) [3]. This was particularly evident in the Kaplan-Meier analysis [3].
Increasing evidence indicates the significant role of atrial mechanism in the pathophysiology and prognosis of Takotsubo patients. Backhaus et al evaluated atrial involvement in patients with Takotsubo cardiomyopathy and its prognostic role using CMR-FT [41]. Regarding adverse clinical events, atrial strain parameters (in particular, atrial reservoir strain) revealed a greater AUC than LV ejection fraction (AUC = 0.69 vs. 0.59) and LA size (AUC = 0.69 vs. 0.62) [41].
Ischemic heart disease
Other data have been used to evaluate LA function in predicting the outcomes after a myocardial infarction [33, 42], such as the onset of atrial fibrillation [33]. The authors showed that atrial longitudinal strain is superior to atrial size and LV diastolic dysfunction in envisaging the development of the arrhythmia [33]. In another study evaluating CMR in 321 post-myocardial infarction patients, Leng et al reported an impairment in left atrial reservoir strain and conduit strain [42]. The addition of atrial strain parameters to traditional MRI predictors of heart attack outcomes provided clinicians with greater prognostic accuracy when predicting major adverse cardiac events (0.75 vs. 0.66, p = 0.04) [42].
Right atrial strain
Both atria have important and different functions during the cardiac cycle. The right atrial (RA) is now being considered as more than a passive chamber and can be subdivided, like the LA strain, into three phases (reservoir, conduit, and booster) [43]. Measurements of RA deformation using STE are limited due to the retrosternal location of the RA, the presence of the caval venous system, and the very thin atrial wall [44, 45]. Conversely, CMR represents the reference standard for RA functional assessments (Fig. 3) [46]. Truong et al evaluated all phases of RA strain using CMR-FT in 61 healthy subjects, reporting mean values of 35.53 ± 14.35, 22.57 ± 11.08, 12.96 ± 6.36, 1.6 (1.3–2.2), −1.7 (−2.4 to −1.3), and −1.5 (−2.1 to −1.1) for reservoir, conduit and booster strain, and strain rate parameters, respectively [45].
Pulmonary arterial hypertension
Many studies have displayed the crucial role of RA function in pulmonary arterial hypertension [47,48,49]. For instance, Hope et al evaluated the functional change in RA in patients with the same disease [47] and reported that RA longitudinal strain was impaired in comparison with healthy subjects. Also of interest, this RA parameter showed a significant difference between compensated and decompensated subgroups of pulmonary hypertension. In addition, RA longitudinal strain showed an excellent AUC (= 0.92) in identifying patients who are at risk of developing future adverse outcomes, with a sensitivity and specificity of 100% and 84.6%, respectively [47].
Ischemic heart disease and cardiomyopathies
The usefulness of RA strain has also been evaluated in other clinical scenarios. Schuster et al investigated its application in 1235 patients after acute myocardial infarction [46]. RA reservoir and conduit parameters showed the most significance in predicting the onset of major adverse cardiovascular events and identifying the degree of heart failure [46]. Furthermore, Dick et al reported that both atria strain parameters were impaired in myocarditis cases [32].
Heart failure with preserved ejection fraction
Studies have also evaluated RA function in HFpEF [50, 51]. Jain et al investigated RA strain (measured with CMR) among patients with HFpEF and evaluated the relationship between RA function and all-cause death. The authors found that RA reservoir and conduit function (but not booster function) were significant predictors of mortality, even after adjusting for confounders, suggesting that RA impairment may be an important cardiac marker in patients with or a risk of heart failure [50]. Also, von Roeder et al tested RA function in patients with HFpEF using CMR compared to invasive hemodynamic measurements. The authors reported that RA conduit function was lower between the control group and HFpEF (p < 0.01), while booster pump increased as compensation (p = 0.01) [51]. Table 2 summarizes the above-stated CMR-FT studies regarding the clinical and prognostic application of atrial strain.
Conclusion
Atrial strain is an emerging parameter with multiple confirmed important clinical and prognostic applications. STE at advanced echocardiography is the most widely used modality for measuring LA strain, given the thinness of the atrial wall. However, CMR-FT, as an alternative modality, offers higher spatial resolution, superior contrast, and greater reproducibility under the same settings. A comprehensive evaluation of the three phases of atrial function by CMR-FT can demonstrate impairments before the onset of atrial enlargement, thus informing clinicians’ decisions and improving patient outcomes.
Change history
22 July 2022
In this article the following funding information was added: Open access funding provided by Università degli Studi di Cagliari within the CRUI-CARE Agreement.
Abbreviations
- CMR:
-
Cardiac magnetic resonance
- FT:
-
Feature tracking
- HFpEF:
-
Heart failure with preserved ejection fraction
- LA:
-
Left atrium or left atrial
- LV:
-
Left ventricle
- RA:
-
Right atrium or right atrial
- SSFP:
-
Steady-state free precession
- ST:
-
Strain rate
- STE:
-
Speckle tracking echocardiography
References
Buggey J, Hoit BD (2018) Left atrial strain: measurement and clinical application. Curr Opin Cardiol 33(5) https://journals.lww.com/co-cardiology/Fulltext/2018/09000/Left_atrial_strain__measurement_and_clinical.5.aspx
Abhayaratna WP, Seward JB, Appleton CP et al (2006) Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 47(12):2357–2363. https://doi.org/10.1016/j.jacc.2006.02.048
Hinojar R, Zamorano JL, Fernández-Méndez MA et al (2019) Prognostic value of left atrial function by cardiovascular magnetic resonance feature tracking in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 35(6):1055–1065. https://doi.org/10.1007/s10554-019-01534-8
Blume GG, Mcleod CJ, Barnes ME et al (2011) Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 12(6):421–430. https://doi.org/10.1093/ejechocard/jeq175
Cau R, Bassareo PP, Mannelli L, Suri JS, Saba L Imaging in COVID-19-related myocardial injury. Int J Cardiovasc Imaging 37(4):1349–1360. https://doi.org/10.1007/s10554-020-02089-9
Cau R, Bassareo P, Saba L (2020) Cardiac involvement in COVID-19—assessment with echocardiography and cardiac magnetic resonance imaging. SN Compr Clin Med 2(7):845–851. https://doi.org/10.1007/s42399-020-00344-7
Csécs I, Yamaguchi T, Kheirkhahan M et al (2020) Left atrial functional and structural changes associated with ablation of atrial fibrillation - cardiac magnetic resonance study. Int J Cardiol. 305:154–160. https://doi.org/10.1016/j.ijcard.2019.12.010
Kowallick JT, Kutty S, Edelmann F et al (2014) Quantification of left atrial strain and strain rate using cardiovascular magnetic resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson. 16(1):60. https://doi.org/10.1186/s12968-014-0060-6
Yang Y, Yin G, Jiang Y, Song L, Zhao S, Lu M (2020) Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson. 22(1):1–11. https://doi.org/10.1186/s12968-019-0589-5
Pedrizzetti G, Claus P, Kilner PJ, Nagel E (2016) Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 18(1):51. https://doi.org/10.1186/s12968-016-0269-7
Zipes DP (1997) Atrial fibrillation. A tachycardia-induced atrial cardiomyopathy. Circulation. 95(3):562–564. https://doi.org/10.1161/01.cir.95.3.562
Goette A, Kalman JM, Aguinaga L et al (2016) EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 18(10):1455–1490. https://doi.org/10.1093/europace/euw161
Scatteia A, Baritussio A, Bucciarelli-Ducci C (2017) Strain imaging using cardiac magnetic resonance. Heart Fail Rev. 22(4):465–476. https://doi.org/10.1007/s10741-017-9621-8
Gan GCH, Ferkh A, Boyd A, Thomas L (2018) Left atrial function: evaluation by strain analysis. Cardiovasc Diagn Ther 8(1):29–46. https://doi.org/10.21037/cdt.2017.06.08
Hoit BD (2017) Evaluation of left atrial function: current status. Struct Hear. 1(3-4):109–120. https://doi.org/10.1080/24748706.2017.1353718
Truong VT, Palmer C, Wolking S et al (2020) Normal left atrial strain and strain rate using cardiac magnetic resonance feature tracking in healthy volunteers. Eur Heart J Cardiovasc Imaging. 21(4):446–453. https://doi.org/10.1093/ehjci/jez157
Mor-Avi V, Lang RM, Badano LP et al (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr 24(3):277–313. https://doi.org/10.1016/j.echo.2011.01.015
Kim DG, Lee KJ, Lee S et al (2009) Feasibility of two-dimensional global longitudinal strain and strain rate imaging for the assessment of left atrial function: a study in subjects with a low probability of cardiovascular disease and normal exercise capacity. Echocardiography. 26(10):1179–1187. https://doi.org/10.1111/j.1540-8175.2009.00955.x
Cameli M, Caputo M, Mondillo S et al (2009) Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. 7:6. https://doi.org/10.1186/1476-7120-7-6
Cau R, Bassareo P, Deidda M et al (2021) Could CMR tissue-tracking and parametric mapping distinguish between Takotsubo syndrome and acute myocarditis? A pilot study. Acad Radiol 21:S1076-6332(21)00015-5. https://doi.org/10.1016/j.acra.2021.01.009
Cau R, Bassareo P, Cherchi V et al (2020) Early diagnosis of chemotherapy-induced cardiotoxicity by cardiac MRI. Eur J Radiol. 130:109158. https://doi.org/10.1016/j.ejrad.2020.109158
Cau R, Cherchi V, Micheletti G, et al (2021) Potential role of artificial intelligence in cardiac magnetic resonance imaging. J Thorac Imaging. Publish Ah(3):142-148. 10.1097/rti.0000000000000584
Leng S, Tan RS, Zhao X, Allen JC, Koh AS, Zhong L (2018) Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 20(1):1–15. https://doi.org/10.1186/s12968-018-0496-1
Leng S, Tan R-S, Zhao X, Allen JC, Koh AS, Zhong L (2018) Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 20(1):71. https://doi.org/10.1186/s12968-018-0496-1
Pathan F, Zainal Abidin HA, Vo QH et al (2021) Left atrial strain: a multi-modality, multi-vendor comparison study. Eur Hear J - Cardiovasc Imaging. 22(1):102–110. https://doi.org/10.1093/ehjci/jez303
Inoue YY, Alissa A, Khurram IM et al (2015) Quantitative tissue-tracking cardiac magnetic resonance (CMR) of left atrial deformation and the risk of stroke in patients with atrial fibrillation. J Am Heart Assoc 4(4). https://doi.org/10.1161/JAHA.115.001844
Habibi M, Lima JAC, Khurram IM et al (2015) Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circ Cardiovasc Imaging. 8(2):e002769. https://doi.org/10.1161/CIRCIMAGING.114.002769
Li L, Chen X, Yin G et al (2020) Early detection of left atrial dysfunction assessed by CMR feature tracking in hypertensive patients. Eur Radiol. 30(2):702–711. https://doi.org/10.1007/s00330-019-06397-0
Shao G, Cao Y, Cui Y et al (2020) Early detection of left atrial and bi-ventricular myocardial strain abnormalities by MRI feature tracking in normotensive or hypertensive T2DM patients with preserved LV function. BMC Cardiovasc Disord. 20(1):1–12. https://doi.org/10.1186/s12872-020-01469-2
Bernardini A, Camporeale A, Pieroni M et al (2020) Atrial dysfunction assessed by cardiac magnetic resonance as an early marker of fabry cardiomyopathy. JACC Cardiovasc Imaging. 13(10):2262–2264. https://doi.org/10.1016/j.jcmg.2020.05.011
Doerner J, Bunck AC, Michels G, Maintz D, Baeßler B (2018) Incremental value of cardiovascular magnetic resonance feature tracking derived atrial and ventricular strain parameters in a comprehensive approach for the diagnosis of acute myocarditis. Eur J Radiol. 104(May):120–128. https://doi.org/10.1016/j.ejrad.2018.05.012
Dick A, Schmidt B, Michels G, Bunck AC, Maintz D, Baeßler B (2017) Left and right atrial feature tracking in acute myocarditis: a feasibility study. Eur J Radiol. 89:72–80. https://doi.org/10.1016/j.ejrad.2017.01.028
Jiwon K, Brian Y, C. PM et al (2020) Left atrial strain impairment precedes geometric remodeling as a marker of post-myocardial infarction diastolic dysfunction. JACC Cardiovasc Imaging 13(10):2099-2113. https://doi.org/10.1016/j.jcmg.2020.05.041
Lapinskas T, Bučius P, Urbonaitė L et al (2017) Left atrial mechanics in patients with acute STEMI and secondary mitral regurgitation: a prospective pilot CMR feature tracking study. Medicina (B Aires). 53(1):11–18. https://doi.org/10.1016/j.medici.2017.02.001
Bertelsen L, Diederichsen SZ, Haugan KJ et al (2020) Left atrial volume and function assessed by cardiac magnetic resonance imaging are markers of subclinical atrial fibrillation as detected by continuous monitoring. Europace 22(5):724–731. https://doi.org/10.1093/europace/euaa035
Critser PJ, Truong V, Powell AW et al (2021) Cardiac magnetic resonance derived atrial function in patients with a Fontan circulation. Int J Cardiovasc Imaging 37(1):275–284
Steinmetz M, Broder M (2018) Hösch O, et al Atrio-ventricular deformation and heart failure in Ebstein’s anomaly - a cardiovascular magnetic resonance study. Int J Cardiol. 257:54–61. https://doi.org/10.1016/j.ijcard.2017.11.097
von Roeder M, Rommel K-P, Kowallick JT et al (2017) Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 10(4):e005467. https://doi.org/10.1161/CIRCIMAGING.116.005467
Chirinos JA, Sardana M, Ansari B et al (2018) Left atrial phasic function by cardiac magnetic resonance feature tracking is a strong predictor of incident cardiovascular events. Circ Cardiovasc Imaging. 11(12):e007512. https://doi.org/10.1161/CIRCIMAGING.117.007512
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39.e14. https://doi.org/10.1016/j.echo.2014.10.003
Backhaus SJ, Stiermaier T, Lange T et al (2019) Atrial mechanics and their prognostic impact in Takotsubo syndrome: a cardiovascular magnetic resonance imaging study. Eur Hear J Cardiovasc Imaging 20(9):1059–1069. https://doi.org/10.1093/ehjci/jey219
Leng S, Ge H, He J et al (2020) Long-term prognostic value of cardiac MRI left atrial strain in ST-segment elevation myocardial infarction. Radiology. 296(2):299–309. https://doi.org/10.1148/radiol.2020200176
Wright LM, Dwyer N, Wahi S, Marwick TH (2018) Association with right atrial strain with right atrial pressure: an invasive validation study. Int J Cardiovasc Imaging. 34(10):1541–1548. https://doi.org/10.1007/s10554-018-1368-3
Rudski LG, Lai WW, Afilalo J et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–688. https://doi.org/10.1016/j.echo.2010.05.010
Truong VT, Palmer C, Young M et al (2020) Right atrial deformation using cardiovascular magnetic resonance myocardial feature tracking compared with two-dimensional speckle tracking echocardiography in healthy volunteers. Sci Rep. 10(1):1–7. https://doi.org/10.1038/s41598-020-62105-9
Schuster A, Backhaus SJ, Stiermaier T et al (2020) Impact of right atrial physiology on heart failure and adverse events after myocardial infarction. J Clin Med. 9(1):210. https://doi.org/10.3390/jcm9010210
Hope KD, Calderón Anyosa RJC, Wang Y et al (2018) Right atrial mechanics provide useful insight in pediatric pulmonary hypertension. Pulm Circ. 8(1):2045893218754852. https://doi.org/10.1177/2045893218754852
Querejeta Roca G, Campbell P, Claggett B, Solomon SD, Shah AM (2015) Right atrial function in pulmonary arterial hypertension. Circ Cardiovasc Imaging. 8(11):e003521–e003521. https://doi.org/10.1161/CIRCIMAGING.115.003521
Sakata K, Uesugi Y, Isaka A et al (2016) Evaluation of right atrial function using right atrial speckle tracking analysis in patients with pulmonary artery hypertension. J Echocardiogr. 14(1):30–38. https://doi.org/10.1007/s12574-015-0270-4
Jain S, Kuriakose D, Edelstein I et al (2019) Right atrial phasic function in heart failure with preserved and reduced ejection fraction. JACC Cardiovasc Imaging. 12(8 Pt 1):1460–1470. https://doi.org/10.1016/j.jcmg.2018.08.020
von Roeder M, Kowallick JT, Rommel K-P et al (2020) Right atrial–right ventricular coupling in heart failure with preserved ejection fraction. Clin Res Cardiol. 109(1):54–66. https://doi.org/10.1007/s00392-019-01484-0
Funding
Open access funding provided by Università degli Studi di Cagliari within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Luca Saba.
Conflict of interest
Pontone G. declared institutional research grant and/or honorarium as speaker from General Electric, Bracco, Medtronic, Bayer, and Heartflow. Other authors have no disclosure.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because it is a review.
Ethical approval
Institutional review board approval was not required because this is a review.
Methodology
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cau, R., Bassareo, P., Suri, J.S. et al. The emerging role of atrial strain assessed by cardiac MRI in different cardiovascular settings: an up-to-date review. Eur Radiol 32, 4384–4394 (2022). https://doi.org/10.1007/s00330-022-08598-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08598-6