Abstract
Objective
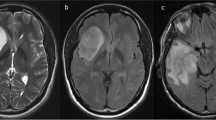
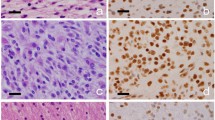
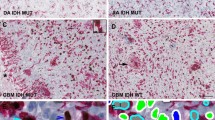
Noninvasive detection of molecular status of astrocytoma is of great clinical significance for predicting therapeutic response and prognosis. We aimed to evaluate whether morphological MRI (mMRI), SWI, DWI, and DSC-PWI could predict Ki-67 labeling index (LI), ATRX mutation, and MGMT promoter methylation status in IDH mutant (IDH-mut) astrocytoma.
Methods
We retrospectively analyzed mMRI, SWI, DWI, and DSC-PWI in 136 patients with IDH-mut astrocytoma.The features of mMRI and intratumoral susceptibility signals (ITSS) were compared using Fisher exact test or chi-square tests. Wilcoxon rank sum test was used to compare the minimum ADC (ADCmin), and minimum relative ADC (rADCmin) of IDH-mut astrocytoma in different molecular markers status. Mann–Whitney U test was used to compare the rCBVmax of IDH-mut astrocytoma with different molecular markers status. Receiver operating characteristic curves was performed to evaluate their diagnostic performances.
Results
ITSS, ADCmin, rADCmin, and rCBVmax were significantly different between high and low Ki-67 LI groups. ITSS, ADCmin, and rADCmin were significantly different between ATRX mutant and wild-type groups. Necrosis, edema, enhancement, and margin pattern were significantly different between low and high Ki-67 LI groups. Peritumoral edema was significantly different between ATRX mutant and wild-type groups. Grade 3 IDH-mut astrocytoma with unmethylated MGMT promoter was more likely to show enhancement compared to the methylated group.
Conclusions
mMRI, SWI, DWI, and DSC-PWI were shown to have the potential to predict Ki-67 LI and ATRX mutation status in IDH-mut astrocytoma. A combination of mMRI and SWI may improve diagnostic performance for predicting Ki-67 LI and ATRX mutation status.
Clinical relevance statement
Conventional MRI and functional MRI (SWI, DWI, and DSC-PWI) can predict Ki-67 expression and ATRX mutation status of IDH mutant astrocytoma, which may help clinicians determine personalized treatment plans and predict patient outcomes.
Key Points
• A combination of multimodal MRI may improve the diagnostic performance to predict Ki-67 LI and ATRX mutation status.
• Compared with IDH-mutant astrocytoma with low Ki-67 LI, IDH-mutant astrocytoma with high Ki-67 LI was more likely to show necrosis, edema, enhancement, poorly defined margin, higher ITSS levels, lower ADC, and higher rCBV.
• ATRX wild-type IDH-mutant astrocytoma was more likely to show edema, higher ITSS levels, and lower ADC compared to ATRX mutant IDH-mutant astrocytoma.



Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- ADCmin :
-
Minimum apparent diffusion coefficient
- AIC:
-
Akaike information criterion
- ATRX:
-
X-linked alpha-thalassemia/mental retardation syndrome
- AUC:
-
Area under the curve
- CBV:
-
Cerebral blood volume
- CNS:
-
Central nervous system
- DSC-PWI:
-
Dynamic susceptibility contrast perfusion-weighted imaging
- DWI:
-
Diffusion-weighted imaging
- FLAIR:
-
Fluid-attenuated inversion recovery imaging
- FOV:
-
Field of view
- Gd-BOPTA:
-
Gadobenate dimeglumine
- IDH:
-
Isocitrate dehydrogenase
- ITSS:
-
Intratumoral susceptibility signal intensity
- LI:
-
Labeling index
- MGMT:
-
Oxygen-6-methylguanine-DNA-methyltransferase
- MIP:
-
Minimum intensity projection
- mMRI:
-
Morphological magnetic resonance imaging
- rADCmin :
-
Minimum relative apparent diffusion coefficient
- rCBVmax :
-
Relative maximum cerebral blood volume
- ROC:
-
Receiver operating characteristic
- ROI:
-
Regions of interest
- Sen:
-
Sensitivity
- Spe:
-
Specificity
- SWI:
-
Susceptibility-weighted imaging
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
- VIF:
-
Variance inflation factor
- WHO:
-
World Health Organization
References
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23:1231–1251
Liu Y, Tang K, Yan W et al (2013) Identifying Ki-67 specific miRNA-mRNA interactions in malignant astrocytomas. Neurosci Lett 546:36–41
Zeng A, Hu Q, Liu Y et al (2015) IDH1/2 mutation status combined with Ki-67 labeling index defines distinct prognostic groups in glioma. Oncotarget 6:30232–30238
Johnson BE, Mazor T, Hong C et al (2014) Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343:189–193
Kannan K, Inagaki A, Silber J et al (2012) Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget 3:1194–1203
Leeper HE, Caron AA, Decker PA, Jenkins RB, Lachance DH, Giannini C (2015) IDH mutation, 1p19q codeletion and ATRX loss in WHO grade II gliomas. Oncotarget 6:30295–30305
Wiestler B, Capper D, Holland-Letz T et al (2013) ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol 126:443–451
Esteller M, Garcia-Foncillas J, Andion E et al (2000) Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343:1350–1354
Chamberlain MC (2014) Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology 82:2147–2148
Cai J, Yang P, Zhang C et al (2014) ATRX mRNA expression combined with IDH1/2 mutational status and Ki-67 expression refines the molecular classification of astrocytic tumors: evidence from the whole transcriptome sequencing of 169 samples samples. Oncotarget 5:2551–2561
Mathur R, Zhang Y, Grimmer MR et al (2020) MGMT promoter methylation level in newly diagnosed low-grade glioma is a predictor of hypermutation at recurrence. Neuro Oncol 22:1580–1590
Bady P, Kurscheid S, Delorenzi M et al (2018) The DNA methylome of DDR genes and benefit from RT or TMZ in IDH mutant low-grade glioma treated in EORTC 22033. Acta Neuropathol 135:601–615
Haase S, Garcia-Fabiani MB, Carney S et al (2018) Mutant ATRX: uncovering a new therapeutic target for glioma. Expert Opin Ther Targets 22:599–613
Carrillo JA, Lai A, Nghiemphu PL et al (2012) Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol 33:1349–1355
Xing Z, Yang X, She D, Lin Y, Zhang Y, Cao D (2017) Noninvasive assessment of IDH mutational status in World Health Organization Grade II and III astrocytomas using DWI and DSC-PWI combined with conventional MR imaging. AJNR Am J Neuroradiol 38:1138–1144
Hyare H, Rice L, Thust S et al (2019) Modelling MR and clinical features in grade II/III astrocytomas to predict IDH mutation status. Eur J Radiol 114:120–127
Zeng Q, Dong F, Shi F, Ling C, Jiang B, Zhang J (2017) Apparent diffusion coefficient maps obtained from high b value diffusion-weighted imaging in the preoperative evaluation of gliomas at 3T: comparison with standard b value diffusion-weighted imaging. Eur Radiol 27:5309–5315
Emblem KE, Nedregaard B, Nome T et al (2008) Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology 247:808–817
Kickingereder P, Sahm F, Radbruch A et al (2015) IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci Rep 5:16238
Zhang J, Peng H, Wang YL et al (2021) Predictive role of the apparent diffusion coefficient and MRI morphologic features on IDH status in patients with diffuse glioma: a retrospective cross-sectional study. Front Oncol 11:640738
Sunwoo L, Choi SH, Park CK et al (2013) Correlation of apparent diffusion coefficient values measured by diffusion MRI and MGMT promoter methylation semiquantitatively analyzed with MS-MLPA in patients with glioblastoma multiforme. J Magn Reson Imaging 37:351–358
Park MJ, Kim HS, Jahng GH, Ryu CW, Park SM, Kim SY (2009) Semiquantitative assessment of intratumoral susceptibility signals using non-contrast-enhanced high-field high-resolution susceptibility-weighted imaging in patients with gliomas: comparison with MR perfusion imaging. AJNR Am J Neuroradiol 30:1402–1408
Choi YS, Ahn SS, Kim DW et al (2016) Incremental prognostic value of ADC histogram analysis over MGMT promoter methylation status in patients with glioblastoma. Radiology 281:175–184
Hempel JM, Bisdas S, Schittenhelm J et al (2017) In vivo molecular profiling of human glioma using diffusion kurtosis imaging. J Neurooncol 131:93–101
Jiang R, Jiang J, Zhao L et al (2015) Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget 6:42380–42393
Li Y, Qian Z, Xu K et al (2017) Radiomic features predict Ki-67 expression level and survival in lower grade gliomas. J Neurooncol 135:317–324
Bolly HMB, Faried A, Hermanto Y et al (2021) Analysis of mutant isocitrate dehydrogenase 1 immunoexpression, Ki-67 and programmed death ligand 1 in diffuse astrocytic tumours: study of single center in Bandung, Indonesia. J Korean Neurosurg Soc 64:100–109
Reuss DE, Sahm F, Schrimpf D et al (2015) ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol 129:133–146
Jiang JS, Hua Y, Zhou XJ et al (2019) Quantitative assessment of tumor cell proliferation in brain gliomas with dynamic contrast-enhanced MRI. Acad Radiol 26:1215–1221
Theresia E, Malueka RG, Pranacipta S et al (2020) Association between Ki-67 labeling index and histopathological grading of glioma in indonesian population. Asian Pac J Cancer Prev 21:1063–1068
Johannessen AL, Torp SH (2006) The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res 12:143–147
Wang XC, Zhang H, Tan Y et al (2014) Combined value of susceptibility-weighted and perfusion-weighted imaging in assessing who grade for brain astrocytomas. J Magn Reson Imaging 39:1569–1574
Yang X, Lin Y, Xing Z, She D, Su Y, Cao D (2021) Predicting 1p/19q codeletion status using diffusion-, susceptibility-, perfusion-weighted, and conventional MRI in IDH-mutant lower-grade gliomas. Acta Radiol 62:1657–1665
Yang X, Xing Z, She D et al (2022) Grading of IDH-mutant astrocytoma using diffusion, susceptibility and perfusion-weighted imaging. BMC Med Imaging 22:105
Grabner G, Kiesel B, Wöhrer A et al (2017) Local image variance of 7 Tesla SWI is a new technique for preoperative characterization of diffusely infiltrating gliomas: correlation with tumour grade and IDH1 mutational status. Eur Radiol 27:1556–1567
Yan R, Haopeng P, Xiaoyuan F et al (2016) Non-Gaussian diffusion MR imaging of glioma: comparisons of multiple diffusion parameters and correlation with histologic grade and MIB-1 (Ki-67 labeling) index. Neuroradiology 58:121–132
Shiroishi MS, Castellazzi G, Boxerman JL et al (2015) Principles of T2 *-weighted dynamic susceptibility contrast MRI technique in brain tumor imaging. J Magn Reson Imaging 41:296–313
Sugahara T, Korogi Y, Kochi M et al (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 171:1479–1486
Server A, Graff BA, Orheim TE et al (2011) Measurements of diagnostic examination performance and correlation analysis using microvascular leakage, cerebral blood volume, and blood flow derived from 3T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in glial tumor grading. Neuroradiology 53:435–447
Price SJ, Green HA, Dean AF, Joseph J, Hutchinson PJ, Gillard JH (2011) Correlation of MR relative cerebral blood volume measurements with cellular density and proliferation in high-grade gliomas: an image-guided biopsy study. AJNR Am J Neuroradiol 32:501–506
Jiao Y, Killela PJ, Reitman ZJ et al (2012) Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 3:709–722
Hempel JM, Schittenhelm J, Klose U et al (2019) In vivo molecular profiling of human glioma: cross-sectional observational study using dynamic susceptibility contrast magnetic resonance perfusion imaging. Clin Neuroradiol 29:479–491
Horbinski C, McCortney K, Stupp R (2021) MGMT promoter methylation is associated with patient age and 1p/19q status in IDH-mutant gliomas. Neuro Oncol 23:858–860
Li WB, Tang K, Zhang W et al (2011) Relationship between magnetic resonance imaging and molecular pathology in patients with glioblastoma multiforme. Chin Med J 124:2589–2592
Bahrami N, Hartman SJ, Chang YH et al (2018) Molecular classification of patients with grade II/III glioma using quantitative MRI characteristics. J Neurooncol 139:633–642
Drabycz S, Roldán G, de Robles P et al (2010) An analysis of image texture, tumor location, and MGMT promoter methylation in glioblastoma using magnetic resonance imaging. Neuroimage 49:1398–1405
Moon WJ, Choi JW, Roh HG, Lim SD, Koh YC (2012) Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: the CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology 54:555–563
Romano A, Calabria LF, Tavanti F et al (2013) Apparent diffusion coefficient obtained by magnetic resonance imaging as a prognostic marker in glioblastomas: correlation with MGMT promoter methylation status. Eur Radiol 23:513–520
Lu J, Li X, Li H (2021) Perfusion parameters derived from MRI for preoperative prediction of IDH mutation and MGMT promoter methylation status in glioblastomas. Magn Reson Imaging 83:189–195
Acknowledgements
We thank Zebin Xiao for his advice on manuscript writing.
Funding
This work was funded by the National Natural Science Foundation of China (No. 82071869); the Leading Project of the Department of Science and Technology of Fujian Province (no. 2020Y0025); Fujian Provincial Health Technology Project (no. 2021QNB006); and Joint Funds for the Innovation of Science and Technology, Fujian Province (no. 2021Y9154).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dairong Cao.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some patients in the present study cohort overlapped with one of our studies which is now published online (https://doi.org/10.1186/s12880-022-00832-3), in which we used diffusion, susceptibility, and perfusion-weighted imaging to grade IDH mutant astrocytoma. As for the advanced MRI part, 107 cases (78.6%) overlapped in these two studies. In the present study, we used conventional MRI, SWI, DWI, and DSC-PWI to predict Ki-67 Labeling Index, ATRX Mutation, and MGMT Promoter Methylation Status, which is far different from our previous study.
Methodology
-
retrospective
-
diagnostic or prognostic study
-
performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Hu, C., Xing, Z. et al. Prediction of Ki-67 labeling index, ATRX mutation, and MGMT promoter methylation status in IDH-mutant astrocytoma by morphological MRI, SWI, DWI, and DSC-PWI. Eur Radiol 33, 7003–7014 (2023). https://doi.org/10.1007/s00330-023-09695-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09695-w