Abstract
In this work, the impact of La doping on structural and magnetic properties of Ca1-xLaxFe0.5Mn0.5O3-δ (0.05 ≤ x ≤ 0.15) elaborated using the solid-state method has been investigated in details by X-ray diffraction and magnetic measurements. Structural analysis showed that all the phases crystallize in the cubic system with a Pm-3m space group. The electron density (ED) study revealed that Mn/Fe-O and Ca/La-O form partial covalent and ionic bonds, respectively, in the unit cell. Importantly, the ED plots show also the formation of an alternate ordered arrangement of oxygen elements and their vacancies. The magnetic study revealed that all our investigated phases exhibit a paramagnetic (PM)-antiferromagnetic (AFM) transition. The decrease in Neel temperature (TN) with increasing x can be explained by the enhancement of ferromagnetic (FM) magnetic interactions due to La doping. Non-null magnetization and the magnetic hysteresis loop at room temperature confirm the presence of weak ferromagnetism in the PM region. These materials also have complex magnetic responses below TN, which are related to the formation of a variety of magnetic exchange interactions within the system, including AFM, FM and ferrimagnetic (FiM) ordering. As a result, exchange coupling between FM/FiM moments and the antiphase boundaries of AFM explains the observed exchange bias in the compounds. Given the practical applications of perovskites, the current observation of the exchange bias effect and ambient temperature ferromagnetism in these materials may be of major technological significance.
Graphical Abstract

Article Highlights
-
Ca1-xLaxFe0.5Mn0.5O3-δ (x = 0.05, 0.1 and 0.15) ceramics are elaborated.
-
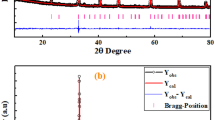
XRD patterns of all compounds are indexed in the Pm-3 m space group.
-
An alternate arrangement of oxygen elements and their vacancies.
-
Magnetic measurements indicate the formation of weak ferromagnetism in the paramagnetic (PM) region in the system.
-
All the samples exhibit PM-AFM phase transition and the Neel temperature (TN) decreases with Ca content.
-
Complex magnetic responses below TN induced the exchange bias.












Similar content being viewed by others
References
Y. Xu, M. Meier, P. Das, M.R. Koblischka, U. Hartmann, Perovskite manganites: potential materials for magnetic cooling at or near room temperature. Cryst. Eng. 5, 383–389 (2002). https://doi.org/10.1016/S1463-0184(02)00049-7
A. Rostamnejadi, M. Venkatesan, P. Kameli, H. Salamati, J.M.D. Coey, Magnetocaloric effect in manganite above room temperature. J. Magn. Magn. Mater. 323, 2214–2218 (2011). https://doi.org/10.1016/j.jmmm.2011.03.036
M.D. Daivajna, A. Rao, G.S. Okram, Electrical, thermal and magnetic studies on Bi-substituted LSMO manganites. J. Magn. Magn. Mater. 388, 90–95 (2015). https://doi.org/10.1016/j.jmmm.2015.04.025
Y. Yevilevich, L. Vradman, J. Zana, I.A. Weinstock, M. Herskowitz, Investigation into La(Fe/Mn)O3 perovskites formation over time during molten salt synthesis. J. Inorg. Chem. 61(17), 6367–6375 (2022). https://doi.org/10.1021/acs.inorgchem.1c03280
Y. Meng, H. Fan, Z. Lu, X. Zhong, J. Lu, Y. Ou, L. Zhou, Mn4+-doped La(CaZr)0.5O3 phosphors for plant grow LEDs: Ab initio site occupy and photoluminescence properties. J. Mater. Res. Bull. 147, 111610 (2022)
N. Nath, S. Chakroborty, P. Panda, K. Pal, High yield silica-based emerging nanoparticles activities for hybrid catalyst applications. Top Catal. (2022). https://doi.org/10.1007/s11244-022-01623-4
S. Wang, P. Han, Y. Zhao, W. Sun, R. Wang, X. Jiang, C. Wu, C. Sun, H. Wei, Oxygen-vacancy-mediated LaFe1-xMnxO3-δ perovskite nanocatalysts for degradation of organic pollutants through enhanced surface ozone adsoption and metal doping effects. Nanoscale 13(30), 12874–12884 (2021). https://doi.org/10.1039/D1NR03055H
K. Pal, A.A. Aljabali, S. Kralj, S. Thomas, F. Gomes de Souzaf, Graphene-assembly liquid crystalline and nanopolymer hybridization: a review on switchable device implementations. Chemosphere 263, 128104 (2021). https://doi.org/10.1016/j.chemosphere.2020.128104
K. Pal, A. Si, G.S. El-Sayyad, M.A. Elkodous, R. Kumar, A.I. El-Batal, S. Kralj, S. Thomas, Cutting edge development on graphene derivatives modified by liquid crystal and CdS/TiO2 hybrid matrix: optoelectronics and biotechnological aspects. Crit. Rev. Solid State Mater. Sci. 46(5), 385–449 (2021). https://doi.org/10.1080/10408436.2020.1805295
T. Ishihara, S. Wang, K.-T. Wu, Highly active oxide cathode of La(Sr)Fe(Mn)O3 for intermediate temperature CO2 and CO2-H2O co-electrolysis using LSGM electrolyte. Solid State Ion. 299, 60–63 (2017). https://doi.org/10.1016/j.ssi.2016.09.013
P. Lalitha, A. Sinthiya, S. Vallinayagam, K. Pal, Growth dynamics and spectroscopic characterization strategies of ‘L-proline’ doped potassium hydrogen phthalate single crystal structural avenue. J. Mol Struct. 1249, 131647 (2022)
O.M. Ama, U.O. Aigbe, W.W. Anku, O.A. Osibote, K. Pal, Degradation of methylene blue dye and bisphenol-a using expanded graphene-polypyrrole-magnetite nanocomposite. Top Catal. (2022). https://doi.org/10.1007/s11244-022-01626-1
Y. Zhang, J. Zhang, B. Chen, J. Zhang, D. Fu, W. Wang, Magnetic field effect on the dielectric behavior in SrRuO3/Ba(Zr0.3Ti0.7)O3/FeMn/Ba(Zr0.3Ti0.7)O3/n-Si heterostructure. Thin Solid Films 749, 139182 (2022). https://doi.org/10.1016/j.tsf.2022.139182
M. Rosić, M. Logar, J. Zagorac, A. Devečerski, A. Egelja, V. Kusigerski, V. Spasojević, B. Matović, Investigation of the structure and the magnetic behavior of nanostructure Ca1-xGdxMnO3 (x = 0.05; 0.1; 0.15; 0.2) obtained by modified glycine nitrate procedure. J. Ceram. Int. 39, 1853–1861 (2013). https://doi.org/10.1016/j.ceramint.2012.08.033
J.J. Neumeier, D.H. Goodwin, Unusually strong ferromagnetic correlations in La-doped CaMnO3. J. Appl. Phys. 85, 5591 (1999). https://doi.org/10.1063/1.369809
A. Bhaskar, C.J. Liu, J.J. Yuan, Thermoelectric properties of Ca1-xGdxMnO3-δ (0.00, 0.02, and 0.05) systems. Sci. World J. (2012). https://doi.org/10.1100/2012/1496.70
Y. Dai, H. Li, Y. Wang, K. Zhong, H. Zhang, J. Yu, Z. Huang, J. Yan, L. Huang, X. Liu, Y. Lu, T. Xu, M. Su, Zn-doped CaFeO3 perovskite-derived high performed catalyst on oxygen reduction reaction in microbial fuel cells. J. Power Sources. 489, 229498 (2021). https://doi.org/10.1016/j.jpowsour.2021.229498
Q. Sun, Z. Yang, The charge disproportionation and electric properties of perovskite CaFe1-xCoxO3. Results Phys. 24, 104198 (2021). https://doi.org/10.1016/j.rinp.2021.104198
M. Tadić, D. Marković, M. Panjan, V. Spasojević, Solution combustion synthesis method and magnetic properties of synthesized polycrystalline calcium manganite CaMnO3-δ powder. J. Ceram. Int. 42, 19365–19371 (2016)
P.M. Woodward, D.E. Cox, E. Moshopoulou, A.W. Sleight, S. Morimoto, Structural studies of charge disproportionation and magnetic order in CaFeO3. J. Phys. Rev. B. 62(2), 844 (2000). https://doi.org/10.1103/PhysRevB.62.844
M. Takano, N. Nakanishi, Y. Takeda, S. Naka, T. Takada, Charge disproportionation in CaFeO3 studied with the Mössbauer effect. Mater. Res. Bull. 12, 923–928 (1977). https://doi.org/10.1016/0025-5408(77)90104-0
B. Ghosh, K. Bagani, M.K. Ray, M. Sardar, S. Banerjee, Re-entrant magnetic phases in CaFeO3 nanoparticle. J. AIP Proc. 1536, 943–944 (2013)
Y. Hosaka, N. Ichikawa, T. Saito, J.P. Attfield, Y. Shimakawa, Charge and spin order in the perovskite CaFe0.5Mn0.5O3: Charge disproportionation behavior of randomly arranged Fe4+. J. Phys. Rev. B. 94, 104429 (2016)
N.N. Loshkareva, A.V. Korolev, N.I. Solin, E.V. Mostovshchikova, S.V. Naumov, N.V. Kostromitina, A.M. Balbashov, Magnetic, electrical and optical properties of Ca1-xCexMnO3 (x ≤ 0.12) single crystals. J. Exp. Theor. Phys. 108, 88–97 (2009). https://doi.org/10.1134/S1063776109010129
J. Zagorac, S. Bošković, B. Matović, B. Babić-Stojić, Structure and magnetic investigations of Ca1-xYxMnO3 (x=0, 0.1, 0.2, 0.3) and Mn4+/Mn3+ relation analysis. Sci. Sinter. 42, 221–232 (2010). https://doi.org/10.2298/SOS1002221Z
C. Chiorescu, J.J. Neumeier, J.L. Cohn, Cohn, Magnetic inhomogeneity and magnetotransport in electron-doped Ca1-xLaxMnO3 (0 ≤ x ≤ 0.10). Phys. Rev. B. 73, 014406 (2006). https://doi.org/10.1103/PhysRevB.73.014406
B.T. Cong, T. Tsuji, P.X. Thao, P.Q. Thanh, Y. Yamamura, Physica B. 352, 18–23 (2004). https://doi.org/10.1016/j.physb.2004.06.033
C. Martin, A. Maignan, M. Hervieu, B. Raveau, Magnetic phase diagrams of Ln1KxAxMnO3 manganites. Phys. Rev. B. 60, 12191 (1999). https://doi.org/10.1103/PhysRevB.60.12191
T. Esaka, M. Kamata, M. Ohnishi, Control of oxygen deficiency in Cal-xLaxMnO3-δ cathodic properties in alkaline solution. J. Appl. Electrochem. 26, 439–442 (1996). https://doi.org/10.1007/BF00251330
T. Esaka, H. Morimoto, H. Iwahara, Nonstoichiometry in perovskite-type oxide Ca1-xCexMnO3-δ and its properties in alkaline solution. J. Appl. Electrochem. 22, 821–824 (1992). https://doi.org/10.1007/BF01023724
J. Zagorac, S. Bošković, B. Matović, B. Babić-Stojić, Structure and magnetic investigations of Ca1-xYxMnO3 ( x = 0, 0.1, 0.2, 0.3) and Mn4+/Mn3+ relation analysis. Sci. Sinter. 42, 221–232 (2010). https://doi.org/10.2298/SOS1002221Z
G.L. Liu, J.S. Zhou, J.B. Goodenough, Phys. Rev. B. 64, 144414 (2001). https://doi.org/10.1103/PhysRevB.64.144414
H.M. Rietveld, A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 2, 65 (1969). https://doi.org/10.1107/S0021889869006558
F. Khammassi, W. Cherif, A.J.M. Sales, K. Riahi, M.P.F. Graça, M. Dammak, Conduction Mechanism and Dielectric Properties of Polycrystalline La0.53Ca0.47Mn0.5Cr0.5O3. J. Supercond. Nov. Magn. 34(2), 497–505 (2021). https://doi.org/10.1007/s10948-020-05697-7
M. Khlifi, E. Dhahri, E.K. Hlil, Magnetic, magnetocaloric, magnetotransport and magnetoresistance properties of calcium deficient manganites La0.8Ca0.2-x□xMnO3 post-annealed at 800°C. J. Alloys Compd. 587, 771–777 (2014). 1016/j.jallcom.2013.11.012
R. Rozilah, N. Ibrahim, A.K. Yahya, Inducement of ferromagnetic-metallic phase and magnetoresistance behavior in charged ordered monovalent-doped Pr0.75Na0.25MnO3 manganite by Ni substitution. J. Solid State Sci. 87, 64–80 (2019)
K. Momma, F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011). https://doi.org/10.1107/S0021889811038970
R. Yogamalar, R. Srinivasan, A. Vinu, K. Ariga, X-ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 149, 1919–1923 (2009). https://doi.org/10.1016/j.ssc.2009.07.043
A. Gholizadeh, X-ray peak broadening analysis in LaMnO3+δ nano-particles with rhombohedral crystal structure. J. Adv. Mater. Proc. 3, 71–83 (2015)
H. Shashidharagowda, S.N. Mathad, M.B. Abbigeri, Structural, vibrational and magnetic characterization of copper doped CoMn2O4 nano-particles synthesized by chemical route. Sci. Sinter. 53, 429–444 (2021). https://doi.org/10.2298/SOS2104429S
A. Hadded, J. Massoudi, E. Dhahri, K. Khirouni, B.F.O. Costa, Structural, optical and dielectric properties of Cu1.5Mn1.5O4 spinel nanoparticles. RSC Adv. 10, 42542 (2020). https://doi.org/10.1039/D0RA08405K
Y.M. Abbas, A.B. Mansour, S.E. Ali, A.H. Ibrahim, Investigation of structural and magnetic properties of multiferroic La1-xYxFeO3 Perovskites, prepared by citrate auto-combustion technique. J. Magn. Magn. Mater. 482, 66–74 (2019). https://doi.org/10.1016/j.jmmm.2019.03.056
M.E. Abrishami, M. Risch, J. Scholz, V. Roddatis, N. Osterthun, Ch. Jooss, Oxygen evolution at manganite perovskite Ruddlesden-Popper type particles: trends of activity on structure, valence and covalence. Materials. 9(11), 921 (2016). https://doi.org/10.3390/ma9110921
S. Jaiswar, K.D. Mandal, Evidence of enhanced oxygen vacancy defects inducing ferromagnetism in multiferroic CaMn7O12 manganite with sintering time. J. Phys. Chem. C. 121, 19586–19601 (2017). https://doi.org/10.1021/acs.jpcc.7b05415
I.A. Leonidov, E.I. Konstantinova, M.V. Patrakeev, V.L. Kozhevnikov, Thermodynamic Properties of Nonstoichiometric Oxygen in Manganite Ca0.9Pr0.1MnO3-δ. Russ. J. Phys. Chem. A. 90, 2123–2128 (2016)
H. Wu, Z. Pei, W. Xia, Y. Lu, K. Leng, X. Zhu, Structural, magnetic, dielectric and optical properties of double perovskite Bi2FeCrO6 ceramics synthesized under high press. J. Alloy. Comp. 819, 153007 (2020). https://doi.org/10.1016/j.jallcom.2019.153007
N. Thenmozhi, S. Sasikumar, S. Sonai, R. Saravanan, Electronic structure and chemical bonding in La1-xSrxMnO3 perovskite ceramics. Mater. Res. Express. 4, 046103 (2017). https://doi.org/10.1088/2053-1591/aa6abf
S. Zouari, M.L. Kahn, M. Ellouze, F. Elhalouani, Elhalouani, Effect of iron substitution on the physico-chemical properties of Pr0.6La0.1Ba0.3Mn1-xFexO3 manganites (with 0 ≤ x ≤ 0.3). Eur. Phys. J. Plus. 130, 1–15 (2015). https://doi.org/10.1140/epjp/i2015-15177-2
N. Geetha, V.S. Kumar, D. Prakash, Synthesis and characterization of LaMn1-xFexO3 (x = 0, 0.1, 0.2) by coprecipitation route. J. Phys. Chem. Biophys. 8, 273–278 (2018). https://doi.org/10.4172/2161-0398.1000273
A. Nasri, S. Zouari, M. Ellouze, J.L. Rehspringer, A.-F. Lehlooh, F. Elhalouani, Structural and magnetic properties of Pr0. 6Sr0. 4Mn1-x FexO3 (0 ≤ x ≤ 0.3) manganites oxide prepared by the ball milling method. J. Supercond Nov Magn. 27(2), 443–451 (2014). https://doi.org/10.1007/s10948-013-2282-5
R. Selmi, W. Cherif, L. Fernandez Barquín, M. de la Fuente Rodríguez, L. Ktari, Structure and spin glass behavior in La0.77Mg0.23-x□xMnO3 (0 ≤ x ≤ 0.2) manganites. J. Alloy. Comp.738, 528–539 (2018). https://doi.org/10.1016/j.jallcom.2017.12.189
D. Kumar, A. Banerjee, Coexistence of interacting ferromagnetic clusters and small antiferromagnetic clusters in La0.5Ba0.5CoO3. J. Phys. Condens. Matter. 25, 216005 (2013). https://doi.org/10.1088/0953-8984/25/21/216005
A. Harbi, H. Moutaabbid, Y. Li, C. Renero-Lecuna, M. Fialin, Y. Le Godec, S. Benmokhtar, M. Moutaabbid, The effect of cation disorder on magnetic properties of new double perovskites La2NixCo1-xMnO6 (x = 0.2–0.8). J. Alloy. Comp. 778, 105–114 (2019). https://doi.org/10.1016/j.jallcom.2018.10.360
R.C. Sahoo, D. Paladhi, P. Dasgupta, A. Poddar, R. Singh, A. Das, T.K. Nath, Antisite -disorder driven large exchange bias effect in phase separated La1.5Ca0.5CoMnO6 double perovskite. J. Magn. Magn. Mater. 428, 86–91 (2017)
M. Ziese, AC-susceptibility study of La0.7Ca0.3MnO3 films on LaAlO3 and SrTiO3. J. Magn. Magn. Mater. 320, 263–269 (2008). https://doi.org/10.1016/j.jmmm.2007.05.040
J. Khelifi, A. Tozri, F. Issaoui, E. Dhahri, E.K. Hlil, The influence of disorder on the appearance of Griffiths phase and magnetoresistive properties in (La1-xNdx)2/3(Ca1-ySry)1/3MnO3 oxides. J. Ceram. Int. 40, 1641–1649 (2014). https://doi.org/10.1016/j.ceramint.2013.07.055
S. Banik, I. Das, Effect of A-site ionic disorder on magnetocaloric properties in large band width manganite systems. J. Alloys Compd. 742, 248–255 (2018). https://doi.org/10.1016/j.jallcom.2018.01.295
A. Marzouki-Ajmi, M. Mansouri, W. Cheikhrouhou-Koubaa, M. Koubaa, A. Cheikhrouhou, Structural, Magnetic and Magnetocaloric properties of Vanadium-doped manganites La0.65Ca0.35Mn1-xVxO3 (0 ≤ x ≤ 0.5). J. Magn. Magn. Mater. 433, 209–215 (2017). https://doi.org/10.1016/j.jmmm.2017.01.097
J.A.M. Roosmalen, E.H.P. Cordfunke, A new defect model to describe the oxygen deficiency in perovskite-type oxides. J. Solid State Chem. 93, 212–219 (1991). https://doi.org/10.1016/0022-4596(91)90290-X
H.M. Usama, A. Sharif, M.A. Zubair, M.A. Gafur, S.M. Hoque, Structural transition and its effect in La, Zr co-substituted mono-domain BiFeO3. J. Appl. Phys. 120, 214106 (2016). https://doi.org/10.1063/1.4969047
V. Markovich, I. Fita, A. Wisniewski, R. Puzniak, C. Martin, D. Mogilyansky, G. Jung, G. Gorodetsky, Evolution of magnetic properties of CaMn1-x NbxO3 with Nb-doping. J. Phys. D Appl. Phys. 48, 325003 (2015). https://doi.org/10.1088/0022-3727/48/32/325003
T. Sarkar, S. Elizabeth, P.S. AnilKumar, Electron doping induced exchange bias and cluster glass magnetism in multiferroic Sc0.8Zr0.2MnO3. J. Magn. Magn. Mater. 466, 225–233 (2018). https://doi.org/10.1088/0022-3727/48/32/325003
S. Ghosh, A. Kumar, A. Pal, P. Singh, P. Gupta, Kh. Anand, U.K. Gautam, A.K. Ghosh, S. Chatterjee, Existence of exchange bias and Griffith phase in (Tb1-xCex)MnO3. J. Magn. Magn. Mater. 500, 166261 (2020). https://doi.org/10.1016/j.jmmm.2019.166261
J. Barman, S. Ravi, Magnetization reversal and tunable exchange bias behavior in Mn-substituted NiCr2O4. J. Mater Sci 53, 7187–7198 (2018). https://doi.org/10.1007/s10853-018-2073-2
R.C. Sahoo, D. Paladhi, P. Dasgupta, A. Poddar, R. Singh, A. Das, T.K. Nath, Antisite-disorder driven large exchange bias effect in phase separated La1.5Ca0.5CoMnO6 double perovskite. J. Magn. Magn. Mater. 428, 86–91 (2017). https://doi.org/10.1016/j.jmmm.2016.12.018
K. Laajimi, M. Khlifi, E.K. Hlil, M.H. Gazzah, J. Dhahri, Enhancement of magnetocaloric effect by Nickel substitution in La0. 67Ca0. 33Mn0. 98Ni0. 02O3 manganite oxide. J. Magn. Magn. Mater. 491, 165625 (2019). https://doi.org/10.1016/j.jmmm.2019.165625
S. Thankachan, B.P. Jacob, S. Xavier, E.M. Mohammed, Effect of neodymium substitution on structural and magnetic properties of magnesium ferrite nanoparticles. Phys. Scr. 87, 1–7 (2013). https://doi.org/10.1088/0031-8949/87/02/025701
Acknowledgements
This paper within the framework of collaboration is supported by the Tunisian Ministry of Higher Education and Scientific Research and the Portuguese Ministry of Science, Technology and Higher Education. The authors acknowledge the i3N (UID/CTM/50025/2020) and CICECO-Aveiro Institute of Materials (UID/CTM/50011/2020), financed by FCT/MEC and FEDER under the PT2020 Partnership Agreement. This work is also funded by national funds (OE), through FCT – Fundação para a Ciência e a Tecnologia, I.P., in the scope of the framework contract foreseen in the numbers 4, 5 and 6 of the article 23, of the Decree-Law 57/2016, of August 29, changed by Law 57/2017, of July 19.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selmi, R., Cherif, W., Khammassi, F. et al. Structural and magnetic properties of Ca1-xLaxFe0.5Mn0.5O3-δ (0.05 ≤ x ≤ 0.15) perovskites. Appl. Phys. A 128, 847 (2022). https://doi.org/10.1007/s00339-022-06001-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06001-1