Abstract
Introduction
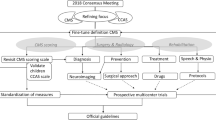
Confusion has surrounded the description of post-operative mutism and associated morbidity in pediatric patients with cerebellar tumors for years. The heterogeneity of definitions and diagnostic features has hampered research progress within the field, and to date, no international guidelines exist on diagnosis, prevention, treatment, or follow-up of this debilitating condition. An international group of clinicians and researchers from multiple relevant disciplines recently formed a cohesive panel to formulate a new working definition and agree upon standardized methods for diagnosis and follow-up.
Methods
Consensus was obtained using the modified nominal group technique, involving four rounds of online Delphi questionnaires interspersed with a structured consensus conference with lectures, group work, and open discussion sessions.
Results
A new, proposed definition of “post-operative pediatric CMS” was formed, preliminary recommendations for diagnostic and follow-up procedures were created, two working groups on a new scoring scale and risk prediction and prevention were established, and areas were identified where further information is needed.
Discussion
The consensus process was motivated by desire to further research and improve quality of life for pediatric brain tumor patients. The Delphi rounds identified relevant topics and established basic agreement, while face-to-face engagement helped resolve matters of conflict and refine terminology. The new definition is intended to provide a more solid foundation for future clinical and research work. It is thought as a consensus for moving forward and hopefully paves the way to developing a standard approach to this challenging problem with the advent of better scoring methods and ultimate goal of reducing the risk of CMS.

Similar content being viewed by others
Notes
International multi-disciplinary group of researchers and health care professionals including adult and pediatric neurosurgeons, adult and pediatric neurologists, (neuro)radiologists, (neuro)psychologists, speech pathologists, linguists and neuroscientists who are active within the field of posterior fossa research.
Speech production that is severely reduced and limited to single words or short sentences that can only be elicited after vigorous stimulation [49].
Impairment of gait (ataxia), extremity coordination (dysmetria), disordered eye movements, poor articulation (dysarthria), impaired swallowing (dysphagia) and tremor [90].
A pattern of behavioral abnormalities that includes impairments of executive function (planning, set-shifting, abstract reasoning, verbal fluency, working memory), often with perseveration, distractibility or inattention; visual-spatial disorganization and impaired visual-spatial memory; personality change with blunting of affect or disinhibited and inappropriate behavior; and difficulties with language production including dysprosodia, agrammatism and mild anomia [35].
Abbreviations
- AM:
-
Akinetic mutism
- CM:
-
Cerebellar mutism
- CCAS:
-
Cerebellar cognitive affective syndrome
- CMS:
-
Cerebellar mutism syndrome
- ISPNO:
-
International Symposium on Pediatric Neuro-Oncology
- MSD:
-
Cerebellar mutism and subsequent dysarthria
- PFS:
-
Posterior fossa syndrome
- R1:
-
Round one (of the Delphi procedure)
- R2:
-
Round two
- R3:
-
Round three
- R4:
-
Round four
- TCM:
-
Transient cerebellar mutism
References
Stiller C (2011) Brain, other CNS and intracranial tumours: 1996-2005. Incidence rates per million population, children (0–14), Great Britain. Available via Cancer Research UK web site. www.cancerresearchuk.org. Accessed 10 Nov 2015
Anonymous (2013) What brain tumors are common in children? Available via Children’s Brain Tumor foundation web site. www.cbtf.org. Accessed 10 Nov 2015
Bonfield CM, Steinbok P (2015) Pediatric cerebellar astrocytoma: a review. Childs Nerv Syst 31:1677–1685
Gudrunardottir T, Lannering B, Remke M, Taylor MD, Wells EM, Keating RF, Packer RJ (2014) Treatment developments and the unfolding of the quality of life discussion in childhood medulloblastoma: a review. Childs Nerv Syst 30:979–990
Michiels EM, Schouten-Van Meeteren AY, Doz F, Janssens GO, van Dalen EC (2015) Chemotherapy for children with medulloblastoma. Cochrane Database Syst Rev 1:CD006678
Packer RJ (2008) Childhood brain tumors: accomplishments and ongoing challenges. J Child Neurol 23:1122–1127
Zacharoulis S, Moreno L (2009) Ependymoma: an update. J Child Neurol 24:1431–1438
Baillieux H, Weyns F, Paquier P, De Deyn PP, Mariën P (2007) Posterior fossa syndrome after a vermian stroke: a new case and review of the literature. Pediatr Neurosurg 43:386–395
Ersahin Y, Mutluer S, Saydam S, Barcin E (1997) Cerebellar mutism: report of two unusual cases and review of the literature. Clin Neurol Neurosurg 99:130–134
Frassanito P, Massimi L, Caldarelli M, Di Rocco C (2009) Cerebellar mutism after spontaneous intratumoral bleeding involving the upper cerebellar vermis: a contribution to the physiopathogenic interpretation. Childs Nerv Syst 25:7–11
Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K (2011) Cerebellar mutism: review of the literature. Childs Nerv Syst 27:355–363
Nedermeijer SC, van den Hout J, Geleijns C, de Klerk H, Catsman-Berrevoets CE (2015) Posterior fossa syndrome in a patient with an ornithine transcarbamylase deficiency. Eur J Paediatr Neurol 19:364–366
Riva D (1998) The cerebellar contribution to language and sequential functions: evidence from a child with cerebellitis. Cortex 34:279–287
Thabet FI, Khalil S, Naz F, Dyme IZ (2013) Cerebellar mutism and reversible cytotoxic edema in influenza B-associated encephalopathy. Pediatr Neurol 49:489–492
Avula S, Mallucci C, Kumar R, Pizer B (2015) Posterior fossa syndrome following brain tumour resection: review of pathophysiology and a new hypothesis on its pathogenesis. Childs Nerv Syst 31:1859–1867
Patay Z (2015) Postoperative posterior fossa syndrome: unraveling the etiology and underlying pathophysiology by using magnetic resonance imaging. Childs Nerv Syst 31:1853–1858
Tamburrini G, Frassanito P, Chieffo D, Massimi L, Caldrelli M, Di Rocco C (2015) Cerebellar mutism. Childs Nerv Syst 31:1841–1851
Daly DD, Love JG (1958) Akinetic mutism. Neurology 8:238–242
Hirsch JF, Renier D, Czernichow P, Benveniste L, Pierre-Kahn A (1979) Medulloblastoma in childhood. Survival and functional results. Acta Neurochir (Wien) 48:1–15
Stein BM, Fraser RA, Tenner MS (1972) Normal pressure hydrocephalus: complication of posterior fossa surgery in children. Pediatrics 49:50–58
Wisoff JH, Epstein FJ (1984) Pseudobulbar palsy after posterior fossa operation in children. Neurosurgery 15:707–709
Rekate HL, Grubb RL, Aram DM, Hahn JF, Ratcheson RA (1985) Muteness of cerebellar origin. Arch Neurol 42:697–698
Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, Dias MS, Allen JC (2006) Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the children’s oncology group. J Neurosurg 105:444–451
Wells EM, Khademian ZP, Walsh KS, Vezina G, Sposto R, Keating RF, Packer RJ (2010) Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr 5:329–334
van Dongen HR, Catsman-Berrevoets CE, van Mourik M (1994) The syndrome of ‘cerebellar’ mutism and subsequent dysarthria. Neurology 44:2040–2046
Kirk EA, Howard VC, Scott CA (1995) Description of posterior fossa syndrome in children after posterior fossa brain tumor surgery. J Pediatr Oncol Nurs 12:181–187
Van Calenbergh F, Van de Laar A, Plets C, Goffin J, Casaer P (1995) Transient cerebellar mutism after posterior fossa surgery in children. Neurosurgery 37:894–898
Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C (1995) Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery 37:885–893
Schmahmann JD, Pandya DN (1987) Posterior parietal projections to the basis pontis in rhesus monkey: possible anatomical substrate for the cerebellar modulation of complex behavior? Neurology 37:291
Schmahmann JD (1991) An emerging concept. The cerebellar contribution to higher function. Arch Neurol 48:1178–1187
Schmahmann JD (1996) From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp 4:174–198
Schmahmann JD (2000) The role of the cerebellum in affect and psychosis. J Neurolinguistics 13:189–214
Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage 44:489–501
Schmahmann JD, Sherman JC (1997) Cerebellar cognitive affective syndrome. Int Rev Neurobiol 41:433–440
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121:561–579
Schmahmann JD (1997) The cerebellum and cognition (editor). International review of neurobiology. Academic Press, San Diego.
Levisohn L, Cronin-Golomb A, Schmahmann JD (2000) Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain 123(Pt 5):1041–1050
Thomale UW, Driever PH (2013) Inconsistent terminology for cerebellar mutism. Childs Nerv Syst 29:717–718
Gudrunardottir T, De Smet H, Bartha-Doering L, Van Dun K, Verhoeven J, Paquier P, Mariën P (2015) Posterior fossa syndrome and cerebellar mutism. In: The Linguistic Cerebellum (ed) P Mariën, M Monti. 1st edn. Academic Press (Elsevier), Oxford, pp. 257–281
Catsman-Berrevoets CE, Van Dongen HR, Mulder PG, Paz y Geuze D, Paquier PF, Lequin MH (1999) Tumour type and size are high risk factors for the syndrome of "cerebellar" mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry 67:755–757
Doxey D, Bruce D, Sklar F, Swift D, Shapiro K (1999) Posterior fossa syndrome: identifiable risk factors and irreversible complications. Pediatr Neurosurg 31:131–136
Avula S, Kumar R, Pizer B, Pettorini B, Abernethy L (2015) Diffusion abnormalities on intraoperative magnetic resonance imaging as an early predictor for the risk of posterior fossa syndrome. Neuro-Oncology 17:614–622
Di Rocco C, Chieffo D, Frassanito P, Caldarelli M, Massimi L, Tamburrini G (2011) Heralding cerebellar mutism: evidence for pre-surgical language impairment as primary risk factor in posterior fossa surgery. Cerebellum 10:551–562
Law N, Greenberg M, Bouffet E, Taylor MD, Laughlin S, Stroher D, Fryer C, McConnell D, Hukin J, Kaise C, Wang F, Mabbott DJ (2012) Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro-Oncology 14:1294–1303
McMillan HJ, Keene DL, Matzinger MA, Vassilyadi M, Nzau M, Ventureyra EC (2009) Brainstem compression: a predictor of postoperative cerebellar mutism. Childs Nerv Syst 25:677–681
Aarsen FK, Arts WF, Van Veelen-Vincent ML, Lequin MH, Catsman-Berrevoets CE (2014) Long-term outcome in children with low grade tectal tumours and obstructive hydrocephalus. Eur J Paediatr Neurol 18:469–474
Ait Khelifa-Gallois N, Laroussinie F, Puget S, Sainte-Rose C, Dellatolas G (2015) Long-term functional outcome of patients with cerebellar pilocytic astrocytoma surgically treated in childhood. Brain Inj 29:366–373
Beckwitt Turkel S, Krieger MD, O’Neil S, Jubran R, Tavare CJ (2012) Symptoms before and after posterior fossa surgery in pediatric patients. Pediatr Neurosurg 48:21–25
Catsman-Berrevoets CE, Aarsen FK (2010) The spectrum of neurobehavioural deficits in the posterior fossa syndrome in children after cerebellar tumour surgery. Cortex 46:933–946
De Smet HJ, Marien P (2012) Posterior fossa syndrome in an adult patient following surgical evacuation of an intracerebellar haematoma. Cerebellum 11:587–592
Grill J, Viguier D, Kieffer V, Bulteau C, Sainte-Rose C, Hartmann O, Kalifa C, Dellatolas G (2004) Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. J Neurosurg 101:152–158
Huber JF, Bradley K, Spiegler B, Dennis M (2007) Long-term neuromotor speech deficits in survivors of childhood posterior fossa tumors: effects of tumor type, radiation, age at diagnosis, and survival years. J Child Neurol 22:848–854
Morgan AT, Liegeois F, Liederkerke C, Vogel AP, Hayward R, Harkness W, Chong K, Vargha-Khadem F (2011) Role of cerebellum in fine speech control in childhood: persistent dysarthria after surgical treatment for posterior fossa tumour. Brain Lang 117:69–76
Moxon-Emre I, Bouffet E, Taylor MD, Laperriere N, Scantlebury N, Law N, Spiegler BJ, Malkin D, Janzen L, Mabbott D (2014) Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol 32:1760–1768
Palmer SL, Hassall T, Evankovich K, Mabbott DJ, Bonner M, Deluca C, Cohn R, Fisher MJ, Morris EB, Broniscer A, Gajjar A (2010) Neurocognitive outcome 12 months following cerebellar mutism syndrome in pediatric patients with medulloblastoma. Neuro-Oncology 12:1311–1317
Puget S, Boddaert N, Viguier D, Kieffer V, Bulteau C, Garnett M, Callu D, Sainte-Rose C, Kalifa C, Dellatolas G, Grill J (2009) Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer 115:1338–1347
Ronning C, Sundet K, Due-Tonnessen B, Lundar T, Helseth E (2005) Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg 41:15–21
Siffert J, Poussaint TY, Goumnerova LC, Scott RM, LaValley B, Tarbell NJ, Pomeroy SL (2000) Neurological dysfunction associated with postoperative cerebellar mutism. J Neuro-Oncol 48:75–81
Steinbok P, Cochrane DD, Perrin R, Price A (2003) Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg 39:179–183
von Hoff K, Kieffer V, Habrand JL, Kalifa C, Dellatolas G, Grill J (2008) Impairment of intellectual functions after surgery and posterior fossa irradiation in children with ependymoma is related to age and neurologic complications. BMC Cancer 8:15
Wells EM, Walsh KS, Khademian ZP, Keating RF, Packer RJ (2008) The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev 14:221–228
Wolfe-Christensen C, Mullins LL, Scott JG, McNall-Knapp RY (2007) Persistent psychosocial problems in children who develop posterior fossa syndrome after medulloblastoma resection. Pediatr Blood Cancer 49:723–726
Callu D, Viguier D, Laroussinie F, Puget S, Boddaert N, Kieffer V, Piana H, Escolano S, Renier D, Sainte-Rose C, Grill J, Dellatolas G (2009) Cognitive and academic outcome after benign or malignant cerebellar tumor in children. Cogn Behav Neurol 22:270–278
Deshmukh VR, Figueiredo EG, Deshmukh P, Crawford NR, Preul MC, Spetzler RF (2006) Quantification and comparison of telovelar and transvermian approaches to the fourth ventricle. Neurosurgery 58:ONS-202-6; discussion ONS-206-7.
El Beltagy MA, Atteya MM (2013) The benefits of navigated intraoperative ultrasonography during resection of fourth ventricular tumors in children. Childs Nerv Syst.
El Beltagy MA, Atteya MM, El-Haddad A, Awad M, Taha H, Kamal M, El Naga SA (2014) Surgical and clinical aspects of cerebellar pilomyxoid-spectrum astrocytomas in children. Childs Nerv Syst 30:1045–1053
El-Bahy K (2005) Telovelar approach to the fourth ventricle: operative findings and results in 16 cases. Acta Neurochir 147:137–142 discussion 142
Han S, Wang Z, Wang Y, Wu A (2013) Transcerebellomedullary fissure approach to lesions of the fourth ventricle: less is more? Acta Neurochir 155:1011–1016
Ojemann JG, Partridge SC, Poliakov AV, Niazi TN, Shaw DW, Ishak GE, Lee A, Browd SR, Geyer RJ, Ellenbogen RG (2013) Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Childs Nerv Syst 29:2071–2077
Rajesh BJ, Rao BR, Menon G, Abraham M, Easwer HV, Nair S (2007) Telovelar approach: technical issues for large fourth ventricle tumors. Childs Nerv Syst 23:555–558
Souweidane MM (2010) Posterior fossa syndrome. J Neurosurg Pediatr 5:325–326 discussion 326-8
Tanriover N, Ulm AJ, Rhoton AL Jr, Yasuda A (2004) Comparison of the transvermian and telovelar approaches to the fourth ventricle. J Neurosurg 101:484–498
Tomasello F, Conti A, Cardali S, La Torre D, Angileri FF (2015) Telovelar approach to fourth ventricle tumors: highlights and limitations. World Neurosurg 83:1141–1147
Zaheer SN, Wood M (2010) Experiences with the telovelar approach to fourth ventricular tumors in children. Pediatr Neurosurg 46:340–343
Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, Marteau T (1998) Consensus development methods, and their use in clinical guideline development. Health Technol Assess 2:i-iv, 1–88.
Ackermann H, Mathiak K, Riecker A (2007) The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum 6:202–213
Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Mariën P (2008) Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg 110:763–773
De Smet HJ, Baillieux H, Catsman-Berrevoets C, De Deyn PP, Mariën P, Paquier PF (2007) Postoperative motor speech production in children with the syndrome of ‘cerebellar’ mutism and subsequent dysarthria: a critical review of the literature. Eur J Paediatr Neurol 11:193–207
Gelabert-Gonzalez M, Fernandez-Villa J (2001) Mutism after posterior fossa surgery. Review of the literature. Clin Neurol Neurosurg 103:111–114
Gordon N (2001) Mutism: elective or selective, and acquired. Brain and Development 23:83–87
Ildan F, Tuna M, Erman T, Göcer AI, Zeren M, Cetinalp E (2002) The evaluation and comparison of cerebellar mutism in children and adults after posterior fossa surgery: report of two adult cases and review of the literature. Acta Neurochir 144:463–473
Küper M, Timmann D (2013) Cerebellar mutism. Brain Lang 127:327–333
Marien P, Engelborghs S, Fabbro F, De Deyn PP (2001) The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang 79:580–600
Mariën P, De Smet HJ, Wijgerde E, Verhoeven J, Crols R, De Deyn PP (2013) Posterior fossa syndrome in adults: a new case and comprehensive survey of the literature. Cortex 49:284–300
Mariën P, De Smet HJ, Paquier P, De Deyn PP, Verhoeven J (2013) Cerebellar mutism. In: Manto M, Gruol DL, Schmahmann J, et al. (eds) Handbook of the cerebellum and cerebellar disorders, 1st, Edition edn. Springer, Dordrecht, pp. 1753–1769
Ozgur BM, Berberian J, Aryan HE, Meltzer HS, Levy ML (2006) The pathophysiologic mechanism of cerebellar mutism. Surg Neurol 66:18–25
Pitsika M, Tsitouras V (2013) Cerebellar mutism. J Neurosurg Pediatr 12:604–614
Reed-Berendt R, Phillips B, Picton S, Chumas P, Warren D, Livingston JH, Hughes E, Morrall MC (2014) Cause and outcome of cerebellar mutism: evidence from a systematic review. Childs Nerv Syst 30:375–385
van Baarsen KM, Grotenhuis JA (2014) The anatomical substrate of cerebellar mutism. Med Hypotheses 82:774–780
Schmahmann JD (2004) Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16:367–378
Pollack IF (1997) Posterior fossa syndrome. Int Rev Neurobiol 41:411–432
Acknowledgments
The Iceland Delphi Group:
Femke Aarsen, Neuropsychologist. Erasmus MC - Sophia Children’s Hospital, Rotterdam, The Netherlands; Shivaram Avula, Radiologist. Alder Hey Children’s NHS Foundation Trust, Liverpool, UK; Kirsten van Baarsen, Neurosurgery Resident. Radboud University Medical Centre, Nijmegen, The Netherlands; Johan Cappelen, Neurosurgeon. St. Olav Hospital HF, Trondheim, Norway; Coriene Catsman-Berrevoets, Pediatric Neurologist. Erasmus MC/Sophia Children’s Hospital, Rotterdam, The Netherlands; Robert Dineen, Radiologist. Queens Medical Centre and the University of Nottingham, UK; Kimberley Docking, Speech Pathologist. University of Sydney, Australia; Jacques Grill, Pediatric Oncologist. Gustave Roussy, University Paris-Sud, Villejuif, France; Pablo Hernáiz Driever, Pediatric Oncologist. Charité-Universitätsmedizin Berlin, Germany; Thora Gudrunardottir, MD, Researcher. North Zealand Hospital, Hilleroed, Denmark; Marianne Juhler, Neurosurgeon. Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark; Caelyn Kaise, Speech-Language Pathologist. Hospital for Sick Children, Toronto, Canada; Robert F. Keating, Pediatric Neurosurgeon. Children’s National Health System, Washington, DC, USA; Mark D. Krieger, Pediatric Neurosurgeon. Children’s Hospital Los Angeles, USA; Ram Kumar, Pediatric Neurologist. Alder Hey Children’s Hospital, Liverpool, UK; Alvaro Lassaletta, Neuro-Oncology Fellow. Hospital for Sick Children, Toronto, Canada; Nicole Law, Psychologist and Neuroscientist. Hospital for Sick Children and the University of Toronto, Canada; Andrew L. Lux, Pediatric Neurologist. Bristol Royal Hospital for Children, Bristol, UK; Donald D. Mabbott, Psychologist. Hospital for Sick Children, Toronto, Canada; Conor Mallucci, Pediatric Neurosurgeon. Alder Hey Children’s Hospital, Liverpool, UK; Angela T. Morgan, Speech Pathologist and Speech Neuroscientist. University of Melbourne and Murdoch Children’s Research Institute, Melbourne, Australia; Iska Moxon-Emre, Doctoral Student in Psychology. Hospital for Sick Children and University of Toronto, Canada. Peter Mariën, Neurolingust. ZNA Middelheim Hospital, Antwerp and Vrije Universiteit, Brussels, Belgium; Roger J. Packer, Pediatric Neurologist. Children’s National Health System, Washington, DC, USA; Philippe Paquier, Neurolinguist. ULB - Hopital Erasme and Vrije Universiteit, Brussels, Belgium; Zoltan Patay, Radiologist. St. Jude Children’s Research Hospital, Memphis, USA; Barry Pizer, Pediatric Oncologist. Alder Hey Children’s Hospital and the University of Liverpool, UK; Stephanie Puget, Pediatric Neurosurgeon. Hôpital Necker-Enfants Malades, Paris, France; Christian Sainte-Rose, Pediatric Neurosurgeon. Hôpital Necker-Enfants Malades, Paris, France; Jeremy D. Schmahmann, Neurologist. Massachusetts General Hospital and Harvard Medical School, Boston, USA; Ulrich-Wilhelm Thomale, Pediatric Neurosurgeon. Charité-Universitätsmedizin Berlin, Germany; Katja von Hoff, Pediatric Oncologist. University Medical Center Hamburg-Eppendorf, Germany; David A. Walker, Pediatric Oncologist. Queen’s Medical Centre and Children’s Brain Tumor Research Centre, Nottingham, UK; Karin S. Walsh, Neuropsychologist. Children’s National Health System, Washington, DC, USA; Elizabeth M. Wells, Pediatric Neurologist. Children’s National Health System, Washington, DC, USA; Jeffrey H. Wisoff, Pediatric Neurosurgeon, New York University Langone Medical Center, New York, USA.
Authors’ contributions
Thora Gudrunardottir initiated the consensus process, acted as facilitator of the Delphi Rounds, and organized the consensus conference. She drafted the initial manuscript, contributed substantially to and revised all versions, and approved it as submitted. Angela T. Morgan served on the consensus committee and applied for funding for the consensus conference. She contributed substantially to and revised all versions of the manuscript. She was responsible for graphics, critically reviewed the final manuscript, and approved it as submitted. Andrew L. Lux served as guarantor of the Delphi Process. He was also group discussion facilitator at the consensus conference and chaired the working group on follow-up. He contributed substantially to and revised all versions of the manuscript and approved it as submitted. David A. Walker served as guarantor of the Delphi Process. He was also group discussion facilitator at the consensus conference and chaired the working group on diagnosis. He contributed substantially to the manuscript, revised and approved it as submitted. Karin S. Walsh served on the consensus committee, contributed to and revised all versions of the manuscript, and approved it as submitted. Elizabeth M. Wells served on the consensus committee, contributed to, revised and approved the manuscript as submitted. Jeffrey H. Wisoff documented the consensus conference and revised and approved the manuscript as submitted. Marianne Juhler served as group discussion facilitator at the consensus conference and chaired the working group on definitions. She contributed to and approved the manuscript as submitted. Jeremy D. Schmahmann contributed substantially to the manuscript, revised and accepted it as submitted. Robert F. Keating served on the consensus committee, contributed substantially to and revised all versions of the manuscript, and accepted it as submitted. Coriene Catsman-Berrevoets served on the consensus committee, contributed substantially to and revised all versions of the manuscript, critically reviewed and accepted it as submitted. All authors participated in the consensus conference. For a list of all participants in the conference by specialty and country, see Appendix III.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding source
No external funding for this manuscript.
Financial disclosure
David Walker is affiliated with the Children’s Brain Tumor Research Centre, Nottingham, UK, which provided partial funding for the consensus conference. The other authors have no financial relationships relevant to this article to disclose.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gudrunardottir, T., Morgan, A.T., Lux, A.L. et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst 32, 1195–1203 (2016). https://doi.org/10.1007/s00381-016-3093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-016-3093-3