Abstract
Purpose
Fructooligosaccharides (FOS) are used as functional foods due to their prebiotic effects. Intestinal anti-inflammatory activity has been established in most, but not all, studies in animal models of colitis, using mainly chemically induced inflammation. Our goal was to test the effect of FOS (degree of polymerization 2–8) in the chronic, lymphocyte-driven CD4+ CD62L+ T cell transfer model of colitis.
Methods
Colitis was induced by transfer of CD4+ CD62L+ T cells to C57BL/6J Rag1−/− mice. FOS (75 mg day−1) was administered by gavage as a post-treatment. Three groups were established: non-colitic (NC), colitic control (C, CD4+ CD62L+ transferred mice treated with vehicle) and colitic+FOS (C+FOS, similar but treated with FOS). Mice were killed after 13 days.
Results
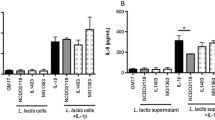
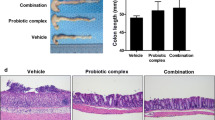
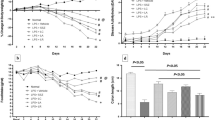
Treatment of mice with FOS ameliorated colitis, as evidenced by an increase in body weight, a lesser myeloperoxidase and alkaline phosphatase activities, a lower secretion of proinflammatory cytokines by mesenteric lymph node cells ex vivo (IFN-γ, IL-17, and TNF-α), and a higher colonic expression of occludin (C+FOS vs. C, p < 0.05). Increased relative abundance of lactic acid bacteria was observed in FOS-treated mice (p < 0.05).
Conclusions
FOS exert intestinal anti-inflammatory activity in T lymphocyte-dependent colitis, suggesting it may be useful in the management of inflammatory bowel disease in appropriate conditions.





Similar content being viewed by others
References
Schirbel A, Fiocchi C (2010) Inflammatory bowel disease: Established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis 11:266–276. doi:10.1111/j.1751-2980.2010.00449.x
Sands BE (2007) Inflammatory bowel disease: past, present, and future. J Gastroenterol 42:16–25
Vogt L, Meyer D, Pullens G, Faas M, Smelt M, Venema K, Ramasamy U, Schols HA, De Vos P (2015) Immunological properties of inulin-type fructans. Crit Rev Food Sci Nutr 55:414–436. doi:10.1080/10408398.2012.656772
Ortega-González M, Ocón B, Romero-Calvo I, Anzola A, Guadix E, Zarzuelo A, Suárez MD, Sánchez de Medina F, Martínez-Augustin O (2014) Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB. Mol Nutr Food Res 58:384–393. doi:10.1002/mnfr.201300296
Vogt LM, Meyer D, Pullens G, Faas MM, Venema K, Ramasamy U, Schols HA, de Vos P (2014) Toll-like receptor 2 activation by beta2–>1-fructans protects barrier function of T84 human intestinal epithelial cells in a chain length-dependent manner. J Nutr 144:1002–1008. doi:10.3945/jn.114.191643
Swennen K, Courtin CM, Delcour JA (2006) Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci Nutr 46:459–471. doi:10.1080/10408390500215746
Roberfroid MB (2005) Introducing inulin-type fructans. Br J Nutr 93(Suppl 1):S13–S25
Nakamura Y, Natsume M, Yasuda A, Ishizaka M, Kawahata K, Koga J (2011) Fructooligosaccharides suppress high-fat diet-induced fat accumulation in C57BL/6J mice. BioFactors. doi:10.1002/biof.147
Hess JR, Birkett AM, Thomas W, Slavin JL (2011) Effects of short-chain fructooligosaccharides on satiety responses in healthy men and women. Appetite 56:128–134
Capitan-Canadas F, Ortega-Gonzalez M, Guadix E, Zarzuelo A, Suarez MD, de Medina FS, Martinez-Augustin O (2014) Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Mol Nutr Food Res 58:1098–1110. doi:10.1002/mnfr.201300497
Cherbut C, Michel C, Lecannu G (2003) The prebiotic characteristics of fructooligosaccharides are necessary for reduction of TNBS-induced colitis in rats. J Nutr 133:21–27
Daddaoua A, Martinez-Plata E, Lopez-Posadas R, Vieites JM, Gonzalez M, Requena P, Zarzuelo A, Suarez MD, de Medina FS, Martinez-Augustin O (2007) Active hexose correlated compound acts as a prebiotic and is antiinflammatory in rats with hapten-induced colitis. J Nutr 137:1222–1228
Fujimori S, Gudis K, Mitsui K, Seo T, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C (2009) A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition 25:520–525
Mitsuyama K, Sata M (2008) Gut microflora: a new target for therapeutic approaches in inflammatory bowel disease. Expert Opin Ther Targets 12:301–312
Daddaoua A, Puerta V, Requena P, Martinez-Ferez A, Guadix E, de Medina FS, Zarzuelo A, Suarez MD, Boza JJ, Martinez-Augustin O (2006) Goat milk oligosaccharides are anti-inflammatory in rats with hapten-induced colitis. J Nutr 136:672–676
Koleva PT, Valcheva RS, Sun X, Ganzle MG, Dieleman LA (2012) Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br J Nutr 108:1633–1643
Rodriguez-Cabezas ME, Camuesco D, Arribas B, Garrido-Mesa N, Comalada M, Bailon E, Cueto-Sola M, Utrilla P, Guerra-Hernandez E, Perez-Roca C, Galvez J, Zarzuelo A (2010) The combination of fructooligosaccharides and resistant starch shows prebiotic additive effects in rats. Clin Nutr 29:832–839
Winkler J, Butler R, Symonds E (2007) Fructo-oligosaccharide reduces inflammation in a dextran sodium sulphate mouse model of colitis. Dig Dis Sci 52:52–58. doi:10.1007/s10620-006-9224-z
Moreau NM, Martin LJ, Toquet CS, Laboisse CL, Nguyen PG, Siliart BS, Dumon HJ, Champ MM (2003) Restoration of the integrity of rat caeco-colonic mucosa by resistant starch, but not by fructo-oligosaccharides, in dextran sulfate sodium-induced experimental colitis. Br J Nutr 90:75–85
Murad-Regadas SM, Souza MH, Brito GA, Rodrigues LV, Regadas FS, Vasconcelos PR (2006) Effect of soluble fiber or fructooligosaccharide supplementation upon trinitrobenzenesulphonic acid induced colitis in rats. Acta Cir Bras 21:315–320
Hedin C, Whelan K, Lindsay JO (2007) Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: a review of clinical trials. Proc Nutr Soc 66:307–315
Benjamin JL, Hedin CR, Koutsoumpas A, Ng SC, McCarthy NE, Hart AL, Kamm MA, Sanderson JD, Knight SC, Forbes A, Stagg AJ, Whelan K, Lindsay JO (2011) Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 60:923–929
Hoentjen F, Welling GW, Harmsen HJ, Zhang X, Snart J, Tannock GW, Lien K, Churchill TA, Lupicki M, Dieleman LA (2005) Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis 11:977–985
Jurjus AR, Khoury NN, Reimund JM (2004) Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods 50:81–92
Martinez-Augustin O, Romero-Calvo I, Suarez MD, Zarzuelo A, Sanchez de Medina F (2009) Molecular bases of impaired water and ion movements in inflammatory bowel diseases. Inflamm Bowel Dis 15:114–127
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL (1989) Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96:795–803
Wirtz S, Neufert C, Weigmann B, Neurath MF (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2:541–546
Solomon L, Mansor S, Mallon P, Donnelly E, Hoper M, Loughrey M, Kirk S, Gardiner K (2010) The dextran sulphate sodium (DSS) model of colitis: an overview. Comp Clin Pathol 19:235–239. doi:10.1007/s00580-010-0979-4
Pizarro TT, Arseneau KO, Bamias G, Cominelli F (2003) Mouse models for the study of Crohn’s disease. Trends Mol Med 9:218–222
Koboziev I, Karlsson F, Zhang S, Grisham MB (2011) Pharmacological intervention studies using mouse models of the inflammatory bowel diseases: translating preclinical data into new drug therapies. Inflamm Bowel Dis 17:1229–1245. doi:10.1002/ibd.21557
Ortega-Gonzalez M, Capitan-Canadas F, Requena P, Ocon B, Romero-Calvo I, Aranda C, Suarez MD, Zarzuelo A, Sanchez de Medina F, Martinez-Augustin O (2014) Validation of bovine glycomacropeptide as an intestinal anti-inflammatory nutraceutical in the lymphocyte-transfer model of colitis. Br J Nutr 111:1202–1212. doi:10.1017/S0007114513003590
Krawisz JE, Sharon P, Stenson WF (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87:1344–1350
Lopez-Posadas R, Gonzalez R, Ballester I, Martinez-Moya P, Romero-Calvo I, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F (2011) Tissue-nonspecific alkaline phosphatase is activated in enterocytes by oxidative stress via changes in glycosylation. Inflamm Bowel Dis 17:543–556. doi:10.1002/ibd.21381
Martinez-Moya P, Ortega-Gonzalez M, Gonzalez R, Anzola A, Ocon B, Hernandez-Chirlaque C, Lopez-Posadas R, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F (2012) Exogenous alkaline phosphatase treatment complements endogenous enzyme protection in colonic inflammation and reduces bacterial translocation in rats. Pharmacol Res 66:144–153
Sanchez de Medina F, Martinez-Augustin O, Gonzalez R, Ballester I, Nieto A, Galvez J, Zarzuelo A (2004) Induction of alkaline phosphatase in the inflamed intestine: a novel pharmacological target for inflammatory bowel disease. Biochem Pharmacol 68:2317–2326
Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB (2009) T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol 296:G135–G146
Barbier M, Cherbut C, Aube AC, Blottiere HM, Galmiche JP (1998) Elevated plasma leptin concentrations in early stages of experimental intestinal inflammation in rats. Gut 43:783–790
Ballinger A, El-Haj T, Perrett D, Turvill J, Obeid O, Dryden S, Williams G, Farthing MJ (2000) The role of medial hypothalamic serotonin in the suppression of feeding in a rat model of colitis. Gastroenterology 118:544–553
Kuo SM, Merhige PM, Hagey LR (2013) The effect of dietary prebiotics and probiotics on body weight, large intestine indices, and fecal bile acid profile in wild type and IL10−/− mice. PLoS ONE 8:e60270. doi:10.1371/journal.pone.0060270
Schijf MA, Kruijsen D, Bastiaans J, Coenjaerts FE, Garssen J, van Bleek GM, van’t Land B (2012) Specific dietary oligosaccharides increase Th1 responses in a mouse respiratory syncytial virus infection model. J Virol 86:11472–11482. doi:10.1128/jvi.06708-11
Delgado GT, Thome R, Gabriel DL, Tamashiro WM, Pastore GM (2012) Yacon (Smallanthus sonchifolius)-derived fructooligosaccharides improves the immune parameters in the mouse. Nutr Res 32:884–892. doi:10.1016/j.nutres.2012.09.012
Anastasovska J, Arora T, Sanchez Canon GJ, Parkinson JR, Touhy K, Gibson GR, Nadkarni NA, So PW, Goldstone AP, Thomas EL, Hankir MK, Van Loo J, Modi N, Bell JD, Frost G (2012) Fermentable carbohydrate alters hypothalamic neuronal activity and protects against the obesogenic environment. Obesity (Silver Spring) 20:1016–1023. doi:10.1038/oby.2012.6
Delzenne NM, Cani PD, Neyrinck AM (2007) Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: experimental data. J Nutr 137:2547s–2551s
Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A (2014) Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 13:3–10. doi:10.1016/j.autrev.2013.06.004
Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T (2011) Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 300:G1054–G1064. doi:10.1152/ajpgi.00055.2011
Acknowledgments
We are grateful to Beneo-Orafti (Tienen, Belgium). This work was funded by Fundación Ramón Areces, by the Ministerio de Economía y Competividad (SAF2008-01432, AGL2008-04332, SAF2011-22922 and SAF2011-22812), and by Junta de Andalucía (CTS164 and CTS6736). BO and CJA are funded by Ministery of Education. AA was funded by Junta de Andalucía. CIBERehd (Centro de Investigación Biomédica en Red, Enfermedades Hepáticas y Digestivas) is funded by the Instituto de Salud Carlos III.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capitán-Cañadas, F., Ocón, B., Aranda, C.J. et al. Fructooligosaccharides exert intestinal anti-inflammatory activity in the CD4+ CD62L+ T cell transfer model of colitis in C57BL/6J mice. Eur J Nutr 55, 1445–1454 (2016). https://doi.org/10.1007/s00394-015-0962-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0962-6