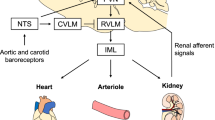
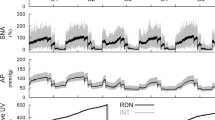
Abstract
Increased cardiac sympathetic nerve activity (CSNA) is a key feature of heart failure (HF) and is associated with poor outcome. There is evidence that central angiotensinergic mechanisms contribute to the increased CSNA in HF, but the central sites involved are unknown. In an ovine, rapid pacing model of HF, we investigated the contribution of the lamina terminalis and area postrema to the increased CSNA and also the responses to fourth ventricular infusion of the angiotensin type 1 receptor antagonist losartan. Ablation of the area postrema or sham lesion (n = 6/group), placement of lamina terminalis lesion electrodes (n = 5), and insertion of a cannula into the fourth ventricle (n = 6) were performed when ejection fraction was ~ 50%. When ejection fraction was < 40%, recording electrodes were implanted, and after 3 days, resting CSNA and baroreflex control of CSNA were measured before and following lesion of the lamina terminalis, in groups with lesion or sham lesion of the area postrema and before and following infusion of losartan (1.0 mg/h for 5 h) into the fourth ventricle. In conscious sheep with HF, lesion of the lamina terminalis did not significantly change CSNA (91 ± 2 vs. 86 ± 3 bursts/100 heart beats), whereas CSNA was reduced in the group with lesion of the area postrema (89 ± 3 to 45 ± 10 bursts/100 heart beats, P < 0.01) and following fourth ventricular infusion of losartan (89 ± 3 to 48 ± 8 bursts/100 heartbeats, P < 0.01). These findings indicate that the area postrema and brainstem angiotensinergic mechanisms may play an important role in determining the increased CSNA in HF.






Similar content being viewed by others
References
Ajijola OA, Hoover DB, Simerly TM, Brown TC, Yanagawa J, Biniwale RM, Lee JM, Sadeghi A, Khanlou N, Ardell JL, Shivkumar K (2017) Inflammation, oxidative stress, and glial cell activation characterize stellate ganglia from humans with electrical storm. JCI Insight 2:94715. https://doi.org/10.1172/jci.insight.94715
Balke CW, Shorofsky SR (1998) Alterations in calcium handling in cardiac hypertrophy and heart failure. Cardiovasc Res 37:290–299. https://doi.org/10.1016/s0008-6363(97)00272-1
Bers DM, Eisner DA, Valdivia HH (2003) Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res 93:487–490. https://doi.org/10.1161/01.RES.0000091871.54907.6B
Bickerton RK, Buckley JP (1961) Evidence for a central mechanism in angiotensin-induced hypertension. Proc Soc Exp Biol Med 106:834–836. https://doi.org/10.3181/00379727-106-26492
Blessing WW, Hedger SC, Joh TH, Willoughby JO (1987) Neurons in the area postrema are the only catecholamine-synthesizing cells in the medulla or pons with projections to the rostral ventrolateral medulla (C1-area) in the rabbit. Brain Res 419:336–340. https://doi.org/10.1016/0006-8993(87)90604-4
Bonham AC, Hasser EM (1993) Area postrema and aortic or vagal afferents converge to excite cells in nucleus tractus solitarius. Am J Physiol 264:H1674–H1685. https://doi.org/10.1152/ajpheart.1993.264.5.H1674
Booth LC, Schlaich MP, Nishi EE, Yao ST, Xu J, Ramchandra R, Lambert GW, May CN (2015) Short-term effects of catheter-based renal denervation on cardiac sympathetic drive and cardiac baroreflex function in heart failure. Int J Cardiol 190:220–226. https://doi.org/10.1016/j.ijcard.2015.03.440
Briest W, Holzl A, Rassler B, Deten A, Leicht M, Baba HA, Zimmer HG (2001) Cardiac remodeling after long term norepinephrine treatment in rats. Cardiovasc Res 52:265–273
Brum PC, Kosek J, Patterson A, Bernstein D, Kobilka B (2002) Abnormal cardiac function associated with sympathetic nervous system hyperactivity in mice. Am J Physiol Heart Circ Physiol 283:H1838–H1845. https://doi.org/10.1152/ajpheart.01063.2001
Cai Y, Hay M, Bishop VS (1996) Synaptic connections and interactions between area postrema and nucleus tractus solitarius. Brain Res 724:121–124
DiBona GF, Jones SY, Brooks VL (1995) ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol 269:R1189–R1196. https://doi.org/10.1152/ajpregu.1995.269.5.R1189
DiBona GF, Kopp UC (1997) Neural control of renal function. Physiol Rev 77:75–197. https://doi.org/10.1152/physrev.1997.77.1.75
Francis J, Wei SG, Weiss RM, Beltz T, Johnson AK, Felder RB (2002) Forebrain-mediated adaptations to myocardial infarction in the rat. Am J Physiol Heart Circ Physiol 282:H1898–H1906. https://doi.org/10.1152/ajpheart.00488.2001
Frithiof R, Ramchandra R, Hood SG, May CN (2011) Hypertonic sodium resuscitation after hemorrhage improves hemodynamic function by stimulating cardiac, but not renal, sympathetic nerve activity. Am J Physiol Heart Circ Physiol 300:H685–H692. https://doi.org/10.1152/ajpheart.00930.2010
Frithiof R, Xing T, McKinley MJ, May CN, Ramchandra R (2014) Intracarotid hypertonic sodium chloride differentially modulates sympathetic nerve activity to the heart and kidney. Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/ajpregu.00460.2013
Gao JQ, Yang W, Liu ZJ (2018) Percutaneous renal artery denervation in patients with chronic systolic heart failure: a randomized controlled trial. Cardiol J. https://doi.org/10.5603/CJ.a2018.0028
Gao L, Wang WZ, Wang W, Zucker IH (2008) Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension 52:708–714. https://doi.org/10.1161/HYPERTENSIONAHA.108.116228
Grassi G, Seravalle G, Bertinieri G, Turri C, Stella ML, Scopelliti F, Mancia G (2001) Sympathetic and reflex abnormalities in heart failure secondary to ischaemic or idiopathic dilated cardiomyopathy. Clin Sci (Lond) 101:141–146
Hasegawa K, Iwai-Kanai E, Sasayama S (2001) Neurohormonal regulation of myocardial cell apoptosis during the development of heart failure. J Cell Physiol 186:11–18. https://doi.org/10.1002/1097-4652(200101)186:1%3c11:AID-JCP1013%3e3.0.CO;2-5
Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI (1986) Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 73:615–621. https://doi.org/10.1161/01.CIR.73.4.615
Hasser EM, Cunningham JT, Sullivan MJ, Curtis KS, Blaine EH, Hay M (2000) Area postrema and sympathetic nervous system effects of vasopressin and angiotensin II. Clin Exp Pharmacol Physiol 27:432–436
Heusch G (2011) Heart rate and heart failure. Not a simple relationship. Circ J 75:229–236
Joy MD, Lowe RD (1970) Evidence that the area postrema mediates the central cardiovascular response to angiotensin II. Nature 228:1303–1304. https://doi.org/10.1038/2281303a0
Kaye DM, Lambert GW, Lefkovits J, Morris M, Jennings G, Esler MD (1994) Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol 23:570–578. https://doi.org/10.1016/0735-1097(94)90738-2
Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD (1995) Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 26:1257–1263. https://doi.org/10.1016/0735-1097(95)00332-0
Li YF, Patel KP (2003) Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand 177:17–26. https://doi.org/10.1046/j.1365-201X.2003.01043.x
Liang C, Rounds NK, Dong E, Stevens SY, Shite J, Qin F (2000) Alterations by norepinephrine of cardiac sympathetic nerve terminal function and myocardial beta-adrenergic receptor sensitivity in the ferret: normalization by antioxidant vitamins. Circulation 102:96–103. https://doi.org/10.1161/01.CIR.102.1.96
Liu JL, Murakami H, Sanderford M, Bishop VS, Zucker IH (1999) ANG II and baroreflex function in rabbits with CHF and lesions of the area postrema. Am J Physiol 277:H342–H350. https://doi.org/10.1152/ajpheart.1999.277.1.H342
Liu JL, Zucker IH (1999) Regulation of sympathetic nerve activity in heart failure: a role for nitric oxide and angiotensin II. Circ Res 84:417–423. https://doi.org/10.1161/01.RES.84.4.417
Mangiapane ML, Simpson JB (1980) Subfornical organ lesions reduce the pressor effect of systemic angiotensin II. Neuroendocrinology 31:380–384. https://doi.org/10.1159/000123107
May CN, McAllen RM, McKinley MJ (2000) Renal nerve inhibition by central NaCl and ANG II is abolished by lesions of the lamina terminalis. Am J Physiol Regul Integr Comp Physiol 279:R1827–R1833. https://doi.org/10.1152/ajpregu.2000.279.5.R1827
McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ (2003) The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172:1–122
Price CJ, Hoyda TD, Ferguson AV (2008) The area postrema: a brain monitor and integrator of systemic autonomic state. Neuroscientist 14:182–194. https://doi.org/10.1177/1073858407311100
Ramchandra R, Hood SG, Denton DA, Woods RL, McKinley MJ, McAllen RM, May CN (2009) Basis for the preferential activation of cardiac sympathetic nerve activity in heart failure. Proc Natl Acad Sci USA 106:924–928. https://doi.org/10.1073/pnas.0811929106
Ramchandra R, Hood SG, Frithiof R, May CN (2009) Discharge properties of cardiac and renal sympathetic nerves and their impaired responses to changes in blood volume in heart failure. Am J Physiol Regul Integr Comp Physiol 297:R665–R674. https://doi.org/10.1152/ajpregu.00191.200
Ramchandra R, Hood SG, Frithiof R, McKinley MJ, May CN (2013) The role of the paraventricular nucleus of the hypothalamus in the regulation of cardiac and renal sympathetic nerve activity in conscious normal and heart failure sheep. J Physiol 591:93–107. https://doi.org/10.1113/jphysiol.2012.236059
Ramchandra R, Hood SG, Watson AM, Allen AM, May CN (2012) Central angiotensin type 1 receptor blockade decreases cardiac but not renal sympathetic nerve activity in heart failure. Hypertension 59:634–641. https://doi.org/10.1161/HYPERTENSIONAHA.111.181131
Ramchandra R, Hood SG, Watson AM, May CN (2008) Responses of cardiac sympathetic nerve activity to changes in circulating volume differ in normal and heart failure sheep. Am J Physiol Regul Integr Comp Physiol 295:R719–R726. https://doi.org/10.1152/ajpregu.00824.2007
Ramchandra R, Watson AM, Hood SG, May CN (2010) Response of cardiac sympathetic nerve activity to intravenous irbesartan in heart failure. Am J Physiol Regul Integr Comp Physiol 298:R1056–R1060. https://doi.org/10.1152/ajpregu.00767.2009
Rundqvist B, Elam M, Bergmann-Sverrisdottir Y, Eisenhofer G, Friberg P (1997) Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation 95:169–175. https://doi.org/10.1161/01.CIR.95.1.169
Ruzicka M, Floras JS, McReynolds AJ, Coletta E, Haddad H, Davies R, Leenen FH (2013) Do high doses of AT(1)-receptor blockers attenuate central sympathetic outflow in humans with chronic heart failure? Clin Sci (Lond) 124:589–595. https://doi.org/10.1042/CS20120437
Schiller AM, Haack KK, Pellegrino PR, Curry PL, Zucker IH (2013) Unilateral renal denervation improves autonomic balance in conscious rabbits with chronic heart failure. Am J Physiol Regul Integr Comp Physiol 305:R886–R892. https://doi.org/10.1152/ajpregu.00269.2013
Shen MJ, Zipes DP (2014) Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 114:1004–1021. https://doi.org/10.1161/CIRCRESAHA.113.302549
Wall KM, Ferguson AV (1992) Endothelin acts at the subfornical organ to influence the activity of putative vasopressin and oxytocin-secreting neurons. Brain Res 586:111–116. https://doi.org/10.1016/0006-8993(92)90580-3
Watson AM, Hood SG, Ramchandra R, McAllen RM, May CN (2007) Increased cardiac sympathetic nerve activity in heart failure is not due to desensitization of the arterial baroreflex. Am J Physiol Heart Circ Physiol 293:H798–H804. https://doi.org/10.1152/ajpheart.00147.2007
Watson AM, Mogulkoc R, McAllen RM, May CN (2004) Stimulation of cardiac sympathetic nerve activity by central angiotensinergic mechanisms in conscious sheep. Am J Physiol Regul Integr Comp Physiol 286:R1051–R1056. https://doi.org/10.1152/ajpregu.00708.2003
Wei SG, Yu Y, Felder RB (2017) Blood-borne interleukin-1beta acts upon the subfornical organ to upregulate the sympathoexcitatory milieu of the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/ajpregu.00211.2017
Xing DT, May CN, Booth LC, Ramchandra R (2014) Tonic arterial chemoreceptor activity contributes to cardiac sympathetic activation in mild ovine heart failure. Exp Physiol 99:1031–1041. https://doi.org/10.1113/expphysiol.2014.079491
Yarbrough WM, Spinale FG (2003) Large animal models of congestive heart failure: a critical step in translating basic observations into clinical applications. J Nucl Cardiol 10:77–86. https://doi.org/10.1067/mnc.2003.16
Yu R, Dickinson CJ (1965) Neurogenic effects of angiotensin. Lancet 2:1276–1277. https://doi.org/10.1016/S0140-6736(65)92288-9
Yu Y, Wei SG, Weiss RM, Felder RB (2017) TNF-alpha receptor 1 knockdown in the subfornical organ ameliorates sympathetic excitation and cardiac hemodynamics in heart failure rats. Am J Physiol Heart Circ Physiol 313:H744–H756. https://doi.org/10.1152/ajpheart.00280.2017
Zhang K, Li YF, Patel KP (2002) Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282:R1006–R1015. https://doi.org/10.1152/ajpregu.00241.2001
Zhang W, Huang BS, Leenen FH (1999) Brain renin-angiotensin system and sympathetic hyperactivity in rats after myocardial infarction. Am J Physiol 276:H1608–H1615. https://doi.org/10.1152/ajpheart.1999.276.5.H1608
Zheng H, Katsurada K, Liu X, Knuepfer MM, Patel KP (2018) Specific afferent renal denervation prevents reduction in neuronal nitric oxide synthase within the paraventricular nucleus in rats with chronic heart failure. Hypertension. https://doi.org/10.1161/HYPERTENSIONAHA.118.11071
Acknowledgements
The authors acknowledge the expert technical assistance of Anthony Dornom and Tom Vale.
Funding
This work was supported by National Health and Medical Research Council of Australia (NHMRC) (APP1128108) and the Victorian Government’s Operational Infrastructure Support Program. L. C. Booth was the recipient of a NHMRC Early Career Fellowship (1054619).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abukar, Y., Ramchandra, R., Hood, S.G. et al. Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res Cardiol 113, 35 (2018). https://doi.org/10.1007/s00395-018-0695-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-018-0695-9