Abstract
Introduction
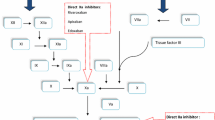
Patients who have undergone hip or knee replacement surgery are exposed to a high risk of developing a post-operative venous thromboembolus and so have a need for an effective, medication-based, thrombosis prophylaxis. New orally active anticoagulants have been available for a few years now. These specific substances directly block either thrombin (e.g., dabigatran etexilate) or Factor Xa (e.g., apixaban). It is not clear whether there are any efficacy differences between these two substances because there have never been any head-to-head studies carried out.
Materials and methods
We have carried out a study comparing two new orally active anticoagulants dabigatran etexilate (Pradaxa®) and apixaban (Eliquis®) that were each given to two groups of 200 patients respectively, who had undergone elective hip or knee arthroplasty (100 each). Each patient was assessed for pre- and post-operative hemoglobin concentrations, post-operative blood loss, the number of transfused erythrocyte concentrates, the duration of wound secretion, clinical thromboembolic complications (deep vein thrombosis, pulmonary embolism, myocardial infarct), as well as gastrointestinal, intracranial or wound-related bleeding complications.
Results
Dabigatran etexilate treatment led to a significant increase in the duration of wound secretion in both arthroplasty groups when compared to apixaban: wound secretion lasted 1.2 days longer on average in the dabigatran etexilate group than in the apixaban group (4.1 ± 2.1 vs. 2.9 ± 1.8 days). There were no significant differences observed between the two anticoagulant groups when comparing pre- and post-operative Hb values, post-operative blood loss and the other clinical parameters.
Conclusions
Thus, it appears that the direct thrombin inhibitor, dabigatran etexilate, is associated with a longer period of wound secretion following the implantation of hip and knee endoprostheses than that associated with the Factor Xa inhibitor, apixaban.
Similar content being viewed by others
References
Adam SS, McDuffie JR, Lachiewicz PF, Ortel TL, Williams JW Jr (2013) Comparative effectiveness of new oral anticoagulants and standard thromboprophylaxis in patients having total hip or knee replacement. Ann Intern Med 159:275–284
Aikens GB, Osmundson JR, Rivey MP (2014) New oral pharmacotherapeutic agents for venous thrombosis prophylaxis after total hip arthroplasty. World J Orthop. 5:188–203
Bonarelli S, Bacchin MR, Frugiuele I, Feoli MA (2015) Dabigatran etexilate and LMWH for the prevention of thromboembolism in 532 patients undergoing hip surgery. Eur Rev Med Pharmacol Sci. 19:897–903
Deitelzweig S (2012) Preventing venous thrombotic events after total hip arthroplasty: new developments in clinical practice. Hosp Pract 40:79–87
Messerschmidt C, Friedman RJ (2015) Clinical experience with novel oral anticoagulants for thromboprophylaxis after elective hip and knee arthroplasty. Arterioscler Trhomb Vasc Biol. 35:771–778
Kwong LM, Kimball JA (2016) Postorthopedic surgery joint replacement surgery venous thromboembolism prophylaxis. Hematol Oncol Clin North Am 30:1007–1018
Feng W, Wu Km Liu Z, Kong G, Deng Z, Chen S, Wu Y, Chen M, Liu S, Wang H (2015) Oral direct factor Xa inhibitor versus enoxaparin for thromboprophylaxis after hip of knee arthroplasty: systematic review, traditional meta-analysis, dose-response meta-analysis and network analysis. Thromb Res 136:1133–1144
Bloch BV, Patel V, Best AJ (2014) Thromboprophylaxis with dabigatran leads to an increased incidence of wound leakage and an increased length of stay after total joint replacement. Bone Joint J 96-B:122–126
Gill SK, Theodorides A, Smith N, Maguire E, Whitehouse SL, Rigby MC, Ivory JP (2011) Wound problems following hip arthroplasty before and after the introduction of a direct thrombin inhibitor for thromboprophylaxis. Hip Int. 21:678–683
Ingelheim Boehringer (2014) Fachinformation Pradaxa® 110 mg Hartkapseln. Boehringer Ingelheim International GmbH, Ingelheim am Rhein, pp 1–19
Bristol-Myers Squibb, Pfizer (2014) Prescribing information Eliquis® 2,5 mg. Bristol-M yers Squibb GmbH & Co, KG aA, München, pp 1–12
Nadler S, Hidalgo J, Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51:224–232
Mercuriali F, Intaglietta M (1996) Proposal of an algorithm to help the choice of the best transfusion strategy. Curr Med Res Opin 13:465–478
Aliyev RM (2010) Total hip replacement using the Staffelstein score: outcome of inpatient rehabilitation. Orthopade. 39:1163–1170
Słupik A, Białoszewski D (2009) A comparative analysis of the clinical utility of the Staffelstein-score and the hospital for special surgery knee score (HSS) in monitoring physiotherapy of total knee replacement patients—preliminary study. Orthop Traumatol Rehabil 11:37–45
Dempfle CE (2012) Pharmocology of the new oral anticoagulants. Herz. 37:362–369
Hanna MS, Mohan P, Knabb R (2014) Annals of the New York Academy of Sciences. Ann N Y Acad Sci 1329:93–106
Völler H, Alban S, Westermann D (2010) New oral anticoagulants: better than Vitamin K-antogonists? Internist. 51:1571–1581
Monroe DM, Hoffman M (2006) What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol 26:41–48
Ansell J (2007) Factor Xa or thrombin: is factor Xa a better target? J Thromb Haemost 5(Suppl-1):60–64
Weitz JI (2012) New oral anticoagulants: a view from the laboratory. Am J Haematol. 87:133–136
Turpie AGG (2007) Oral, direct factor Xa inhibitor in development for the prevention and treatment of the thromboembolic diseases. Arterioscler Thromb Vasc Biol 27(6):1238–1247
Bauer KA (2006) New anticoagulants: anti IIa vs Anti Xa—is one better? J Thromb Thrombolysis 21:67–72
Bauer KA (2011) Recent progress in anticoagulant therapy: oral direct inhibitors of thrombin and factor Xa. J Thromb Haemost 9(Suppl-1):12–19
Konstantinides S, Münzel T (2012) Apixaban. Hämostaseologie. 32:203–211
Wong PC, Pinto DJ, Zhang D (2011) Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis 31:478–492
Ieko M, Tarumi T, Takeda M, Naito S, Nakabayashi T, Koike T (2004) Synthetic selective inhibitors of coagulation factor Xa strongly inhibit thrombin generation without affecting initial thrombin forming time necessary for platelet activation in hemostasis. J Thromb Haemost 2:612–618
Bounameaux H (2009) The novel anticoagulants: entering a new era. Swiss Med Wkly. 139:60–64
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
There is no funding source.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Mayer, A., Schuster, P. & Fink, B. A comparison of apixaban and dabigatran etexilate for thromboprophylaxis following hip and knee replacement surgery. Arch Orthop Trauma Surg 137, 797–803 (2017). https://doi.org/10.1007/s00402-017-2697-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-017-2697-8