Abstract
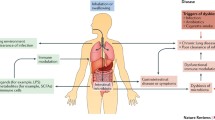
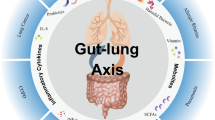
This paper focuses on the gut–lung axis in the context of Inflammatory Bowel Disease (IBD) and Chronic Obstructive Pulmonary Disease (COPD), highlighting the key role played by microbial dysbiosis and the impact of environmental and genetic factors on the innate and acquired immune system and on chronic inflammation in the intestinal and pulmonary tracts. Recent evidence indicates that Antigen-Presenting Cells (APCs) perform regulatory activity influencing the composition of the microbiota. APCs (macrophages, dendritic cells, B cells) possess membrane receptors known as Pattern Recognition Receptors (PRRs), a category of toll-like receptors (TLRs). PRRs recognise distinct microbial structures and microbial metabolites called Signals, which modulate the saprophytic microbial equilibrium of the healthy microbiota by recognising molecular profiles associated with commensal microbes (Microbe-Associated Molecular Patterns, MAMPs). During dysbiosis, pathogenic bacteria can prompt an inflammatory response, producing PAMPs (Pathogen-Associated Molecular Patterns) thereby activating the proliferation of inflammatory response cells, both local and systemic. This series of regulatory and immune-response events is responsible (together with chronic infection, incorrect diet, obesity, etc.) for the systemic chronic inflammation (SCI) known as “low-grade inflammation” typical of COPD and IBD. This review looks at immunological research and explores the role of the microbiota, looking at two recent clinical studies, SPIROMICS and AERIS. There is a need for further clinical studies to characterize the pulmonary microbiota and to obtain new information about the pathogenesis of lung disease to improve our knowledge and treatment strategies and identify new therapeutic targets.

Similar content being viewed by others
References
Black H, Mendoza M, Murin S (2007) Thoracic manifestations of inflammatory bowel disease. Chest 131(2):524–532. https://doi.org/10.1378/chest.06-1074
Global Initiative for Chronic Obstructive Lung Disease (GOLD). http://www.goldcopd.org. Accessed 2021
Vutcovici M, Bitton A, Ernst P, Kezouh A, Suissa S, Brassard P (2016) Inflammatory bowel disease and risk of mortality in COPD. Eur Respir J 47:1357–1364. https://doi.org/10.1183/13993003.01945-2015
Raj AA, Birring SS, Green R, Grant A, de Caestecker J, Pavord ID (2008) Prevalence of inflammatory bowel disease in patients with airways disease. Respir Med 102(5):780–785. https://doi.org/10.1016/j.rmed.2007.08.014
Labarca G, Drake L, Horta G, Jantz MA, Mehta HJ, Fernandez-Bussy S, Folch E, Majid A, Picco M (2019) Association between inflammatory bowel disease and chronic obstructive pulmonary disease: a systematic review and meta analysis. BMC Pulm Med. https://doi.org/10.1186/s12890-019-0963-y
Brassard P, Vutcovici M, Ernst P, Patenaude V, Sewitch M, Suissa S, Bitton A (2015) Increased incidence of inflammatory bowel disease in Québec residents with airway diseases. Eur Respir J 45(4):962–968. https://doi.org/10.1183/09031936.00079414
Lee J, Im JP, Han K, Park S, Soh H, Choi K, Kim J, Chun J, Kim JS (2019) Risk of inflammatory bowel disease in patients with chronic obstructive pulmonary disease: a nationwide, population-based study. World J Gastroenterol 25(42):6354–6364. https://doi.org/10.3748/wjg.v25.i42.6354
Chiu YC, Lee SW, Liu CW, Lan TY, Wu LS (2022) Relationship between gut microbiota and lung function decline in patients with chronic obstructive pulmonary disease: a 1-year follow-up study. Respir Res. https://doi.org/10.1186/s12931-022-01928-8
Chunxi L, Haiyue L, Yanxia L, Jianbing P, Jin S (2020) The gut microbiota and respiratory diseases: new evidence. J Immunol Res 2020:2340670. https://doi.org/10.1155/2020/2340670
Ekbom A, Brandt L, Granath F, Löfdahl CG, Egesten A (2008) Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung 186(3):167–172. https://doi.org/10.1007/s00408-008-9080-z
Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. https://doi.org/10.1128/MMBR.00036-17
Girosi D, Bellodi S, Sabatini F, Rossi GA (2006) The lung and the gut: common origins, close links. Paediatr Respir Rev 7(Suppl 1):S235–S239. https://doi.org/10.1016/j.prrv.2006.04.192
Raftery AL, Tsantikos E, Harris NL, Hibbs ML (2020) Links between inflammatory bowel disease and chronic obstructive pulmonary disease. Front Immunol. https://doi.org/10.3389/fimmu.2020.02144
Kanner RE, Anthonisen NR, Connett JE (2001) Lower respiratory illnesses promote FEV 1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J RespirCrit Care Med 164(3):358–364. https://doi.org/10.1164/ajrccm.164.3.2010017
Vestbo J, Prescott E, Lange P (1996) Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. copenhagen city heart study group. Am J Respir Crit Care Med 153(5):1530–1535. https://doi.org/10.1164/ajrccm.153.5.8630597
Turek EM, Cox MJ, Hunter M, Hui J, James P, Willis-Owen SAG, Cuthbertson L, James A, Musk AW, Moffatt MF, Cookson WOCM (2021) Airway microbial communities, smoking and asthma in a general population sample. EBioMedicine 71:103538. https://doi.org/10.1016/j.ebiom.2021.103538
Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, Delhaes L (2020) The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 10(9):2020. https://doi.org/10.3389/fcimb.2020.00009
Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O (2018) The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol 20(12):e12966. https://doi.org/10.1111/cmi.12966
Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, Bercik P, Verdu EF, McCoy KD, Macpherson AJ (2009) Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 325(5940):617–620. https://doi.org/10.1126/science.1172747
Kuhn KA, Stappenbeck TS (2013) Peripheral education of the immune system by the colonic microbiota. Semin Immunol 25(5):364–369. https://doi.org/10.1016/j.smim.2013.10.002
Borger JG, Lau M, Hibbs ML (2019) The influence of innate lymphoid cells and unconventional T cells in chronic inflammatory lung disease. Front Immunol 10(1597):2019. https://doi.org/10.3389/fimmu.2019.01597
Abels ER, Breakefield XO (2016) Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36(3):301–312. https://doi.org/10.1007/s10571-016-0366-z
Lawson C, Vicencio JM, Yellon D, Davidson SM (2016) Microvesicles and exosomes: new players in metabolicand cardiovascular disease. J Endocrinol 228(2):R57-71. https://doi.org/10.1530/JOE-15-0201
Parkin J, Cohen B (2001) An overview of the immune system. Lancet 357(9270):1777–1789. https://doi.org/10.1016/S0140-6736(00)04904-7
Dommett R, Zilbauer M, George JT, Bajaj-Elliott M (2005) Innate immune defence in the human gastrointestinal tract. Mol Immunol 42(8):903–912. https://doi.org/10.1016/j.molimm.2004.12.004
Gorjifard S, Goldszmid RS (2016) Microbiota-myeloid cell crosstalk beyond the gut. J Leukoc Biol 100(5):865–879. https://doi.org/10.1189/jlb.3RI0516-222R
Gourbal B, Pinaud S, Beckers GJM, Van Der Meer JWM, Conrath U, Netea MG (2018) Innate immune memory: an evolutionary perspective. Immunol Rev 283(1):21–40. https://doi.org/10.1111/imr.12647
McCoy KD, Burkhard R, Geuking MB (2019) The microbiome and immune memory formation. Immunol Cell Biol 97(7):625–635. https://doi.org/10.1111/imcb.12273
Budden KF, Shukla SD, Rehman SF, Bowerman KL, Keely S, Hugenholtz P, Armstrong-James DPH, Adcock IM, Chotirmall SH, Chung KF, Hansbro PM (2019) Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med 7(10):907–920. https://doi.org/10.1016/S2213-2600(18)30510-1
Guo MY, Chen HK, Ying HZ, Qiu FS, Wu JQ (2021) The role of respiratory flora in the pathogenesis of chronic respiratory diseases. Biomed Res Int. https://doi.org/10.1155/2021/6431862
Sulaiman I, Wu BG, Li Y, Tsay JC, Sauthoff M, Scott AS, Ji K, Koralov SB, Weiden M, Clemente JC, Jones D, Huang YJ, Stringer KA, Zhang L, Geber A, Banakis S, Tipton L, Ghedin E, Segal LN (2021) Functional lower airways genomic profiling of the microbiome to capture active microbial metabolism. Eur Respir J. https://doi.org/10.1183/13993003.03434-2020
Magryś A (2021) Microbiota: a missing link in the pathogenesis of chronic lung inflammatory diseases. Pol J Microbiol 70(1):25–32. https://doi.org/10.33073/pjm-2021-013
Pickard JM, Zeng MY, Caruso R, Núñez G (2017) Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev 279(1):70–89. https://doi.org/10.1111/imr.12567
Sekirov I, Russell SL, Antunes CM, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90(3):859–904. https://doi.org/10.1152/physrev.00045.2009
McGuckin MA, Linden SK, Sutton P, Florin TH (2011) Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9(4):265–278. https://doi.org/10.1038/nrmicro2538
Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3(9):710–720. https://doi.org/10.1038/nri1180
Satoh-Takayama N, Kato T, Motomura Y, Kageyama T, Taguchi-Atarashi N, Kinoshita-Daitoku R, Kuroda E, Di Santo JP, Mimuro H, Moro K, Ohno H (2020) Bacteria-induced group 2 innate lymphoid cells in the stomach provide immune protection through induction of IgA. Immunity 52(4):635-649.e4. https://doi.org/10.1016/j.immuni.2020.03.002
Mestecky J, Russell MW (2009) Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of S-IgA at mucosal surfaces. Immunol Lett 124(2):57–62. https://doi.org/10.1016/j.imlet.2009.03.013
Maynard CL, Elson CO, Hatton RD, Weaver CT (2012) Reciprocal interactions of the intestinal microbiota and immune system. Nature 489(7415):231–241. https://doi.org/10.1038/nature11551
Berni Canani R, Paparo L, Nocerino R, Di Scala C, Della Gatta G, Maddalena Y, Buono A, Voto L, Ercolini D (2019) Gut microbiome as target for innovative strategies against food allergy. Front Immunol. https://doi.org/10.3389/fimmu.2019.00191
Latorre E, Layunta E, Grasa L, Pardo J, García S, Alcalde A, Mesonero JE (2018) Toll-like receptors 2 and 4 modulate intestinal IL-10 differently in ileum and colon. United Eur Gastroenterol J 6(3):446–453. https://doi.org/10.1177/2050640617727180
Fung TC, Bessman NJ, Hepworth MR, Kumar N, Shibata N, Kobuley D, Wang K, Ziegler CGK, Goc J, Shima T, Umesaki Y, Sartor RB, Sullivan KV, Lawley TD, Kunisawa J, Kiyono H, Sonnenberg GF (2016) Lymphoid-tissue-resident commensal bacteria promote members of the IL-10 cytokine family to establish mutualism. Immunity 44(3):634–646. https://doi.org/10.1016/j.immuni.2016.02.019
Kotlyarov S, Kotlyarova A (2021) Anti-inflammatory function of fatty acids and involvement of their metabolites in the resolution of inflammation in chronic obstructive pulmonary disease. Int J Mol Sci 22(23):12803. https://doi.org/10.3390/ijms222312803
Vaughan A, Frazer ZA, Hansbro PM, Yang IA (2019) COPD and the gut-lung axis: the therapeutic potential of fibre. J Thorac Dis 11(Suppl 17):S2173–S2180. https://doi.org/10.21037/jtd.2019.10.40
Opron K, Begley LA, Erb-Downward JR, Freeman C, Madapoosi S, Alexis NE, Barjaktarevic I, Graham Barr R, Bleecker ER, Bowler RP, Christenson SA, Comellas AP, Cooper CB, Couper DJ, Doerschuk CM, Dransfield MT, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kaner RJ, Krishnan J, O’Neal WK, Ortega VE, Paine R 3rd, Peters SP, Michael Wells J, Woodruff PG, Martinez FJ, Curtis JL, Huffnagle GB, Huang YJ (2021) Lung microbiota associations with clinical features of COPD in the SPIROMICS cohort. NPJ Biofilms Microbiomes 7(1):14. https://doi.org/10.1038/s41522-021-00185-9
Garcia-Nuñez M, Millares L, Pomares X, Ferrari R, Pérez-Brocal V, Gallego M, Espasa M, Moya A, Monsó E (2014) Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J Clin Microbiol 52(12):4217–4223. https://doi.org/10.1128/JCM.01967-14
Budden KF, Shukla SD, Rehman SF, Bowerman KL, Keely S, Hugenholtz P, Hansbro PM (2019) Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med 7(10):907–920. https://doi.org/10.1016/S2213-2600(18)30510-1
Toraldo DM, Conte L (2019) Influence of the lung microbiota dysbiosis in chronic obstructive pulmonary disease exacerbations: the controversial use of corticosteroid and antibiotic treatments and the role of eosinophils as a disease marker. J Clin Med Res 11(10):667–675. https://doi.org/10.14740/jocmr3875
Herath SC, Normansell R, Maisey S, Poole P (2018) Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009764.pub3
Bourne S, Cohet C, Kim V, Barton A, Tuck A, Aris E, Mesia-Vela S, Devaster JM, Ballou WR, Clarke SC, Wilkinson T (2014) Acute exacerbation and respiratory InfectionS in COPD (AERIS): protocol for a prospective, observational cohort study. BMJ Open 4(3):e004546. https://doi.org/10.1136/bmjopen-2013-004546
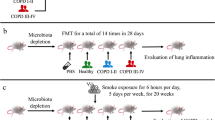
Lai HC, Lin TL, Chen TW, Kuo YL, Chang CJ, Wu TR, Shu CC, Tsai YH, Swift S, Lu CC (2022) Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory parabacteroides goldsteinii lipopolysaccharide. Gut Microbiota 71:309–321. https://doi.org/10.1136/gutjnl-2020-322599
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
FD and DMT provided the idea for the article and wrote the manuscript text; all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Nuccio, F., Piscitelli, P. & Toraldo, D.M. Gut–lung Microbiota Interactions in Chronic Obstructive Pulmonary Disease (COPD): Potential Mechanisms Driving Progression to COPD and Epidemiological Data. Lung 200, 773–781 (2022). https://doi.org/10.1007/s00408-022-00581-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-022-00581-8